 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 32
May 2006
Author

At the Novartis Institutes for BioMedical Research (NIBR) in Basel, Dr. Nicolau Beckmann and his group are dedicated to applying magnetic resonance imaging (MRI) as a non-invasive method to experimental research and pharmacological studies in several disease areas, including Alzheimer’s disease, transplantation, musculoskeletal and airways diseases. The project is based on a close collaboration between the Novartis lab and that of Prof. Clive Page from the Sackler Institute of Pulmonary Pharmacology at King’s College London (University of London). The contribution of many colleagues is acknowledged, who are supporting and contributing to this work: F.X. Blé, C. Cannet, J. Fozard, A. Jackson, H. Karmouty Quintana, L. Mazzoni, E. Schaeublin, R. Sugar, B. Tigani, A. Trifilieff and S. Zurbruegg from Novartis, Prof. N. Frossard from the University of Strasbourg, and Prof. C. Page from King’s College London.
Current address:
Dr. Nicolau Beckmann
nicolau.beckmann@novartis.com
Novartis Pharma AG
Novartis Institutes for BioMedical Research
Postfach
CH-4002 Basel
Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Non-Invasive Methods: Investigation of Airways Diseases by MRI in Rats
To study specific aspects of human diseases, rodents are often used by inducing in them symptoms which resemble those seen in patients. Such animal models serve also for the identification of potential drugs against lung disease[*]. Drug candidates are selected after having shown beneficial effects in in vitro systems. With the present project Nr. 82-02, carried out by Nicolau Beckmann and his team, the 3R Foundation is supporting the MRI approach for measuring lung inflammation and function, and the effects of potential drug therapy. The method allows the health status of individual living animals to be monitored and to reduce the number of animals per experiment by up to 90%.
MRI: a powerful tool
Based on the use of magnetic fields and radiofrequency, MRI basically maps the distribution of hydrogen nuclei (protons) from water and fat in a region of the body. MRI is primarily a clinical diagnostic tool, however, in the past ten years, significant developments have been achieved in imaging small animals as well.
Because of physical characteristics, the living lung is one of the most challenging organs to image by MRI. Using conventional acquisition techniques, the lung appears dark in the images (figure 1). In order to acquire signal from lung parenchyma, special techniques need to be used. A further challenge for lung MRI is that cardiac and respiratory movements may cause marked image artefacts. These problems are more evident in small rodents, because of the higher cardiac and respiratory rates.
For drug testing in vivo, it is important to keep the acquisition conditions as simple as possible so that repeated measurements interfering minimally with the physiology and the well-being of the animals can be carried out on a routine basis. Beckmann et al developed an approach based on a conventional MRI technique, which produces sharp images from the chest of a rat respiring spontaneously (1). The examination time is of approximately 25 min, and during this time the animal is kept anaesthetized by the same anesthetic gas used in the clinic.
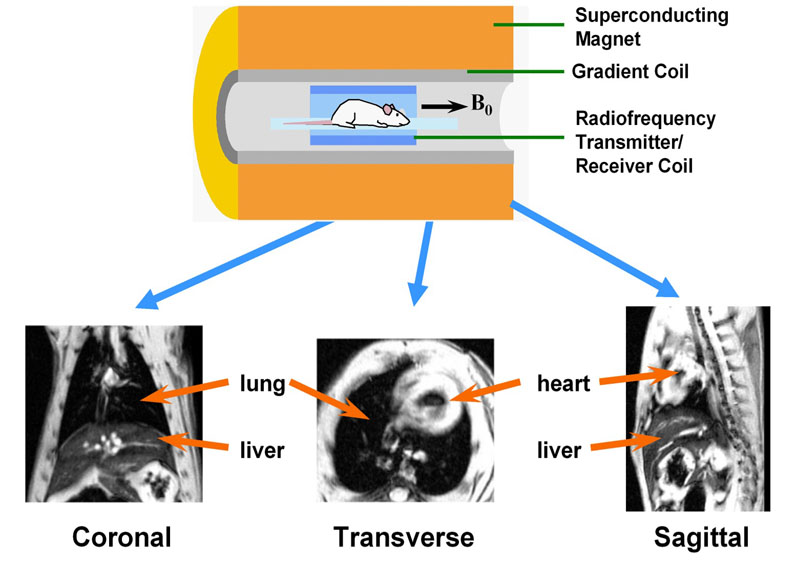
Fig. 1: MRI equipment for small rodent imaging
Essential elements are a magnet (usually superconducting operating at high B0 field, e.g. 4.7 T) generating a homogeneous constant magnetic field, a gradient system capable of producing a position-dependent magnetic field when data acquisition takes place, and a coil which transmits and receives radio-frequency waves. An image is reconstructed from such waves and represents a weighted distribution of water and fat protons in the body. Images with an arbitrary orientation can be obtained using MRI, for instance coronal, transverse, or sagittal sections, as illustrated. Animals are kept under anaesthesia (e.g. with an anaesthetic gas administered through a mask) during the imaging session.
Detection of structural and functional changes
With this approach, effects of inflammation (1), mucus secretion (2), airway and vascular remodeling (3), and parenchymal destruction (4) can be assessed in the rat lung serving as important readouts for models covering a variety of respiratory diseases*. The disease progression is followed in the same animal. The present example serves to illustrate the relevance of the information that can be obtained with MRI.
A characteristic feature of respiratory diseases such as asthma is edema in the airways due to an increased permeability of the lung microvasculature to plasma proteins. Assessment of the fluid leaking out from the microvascular circulation into the surrounding tissue is important for diagnostic purposes. In rats actively sensitized to ovalbumin (OVA) and challenged with the antigen (OVA, 0.3 mg/kg i.t.), an intense, even, fluid signal is detected in the lungs 24 h after challenge (figure 2). Despite the extensive presence of fluid in the lungs, no abnormal behavior by the rats is noticed. The MRI fluid signal that is observed for about 4-5 days, before it resolves spontaneously, significantly correlates with perivascular edema assessed by histology (5).
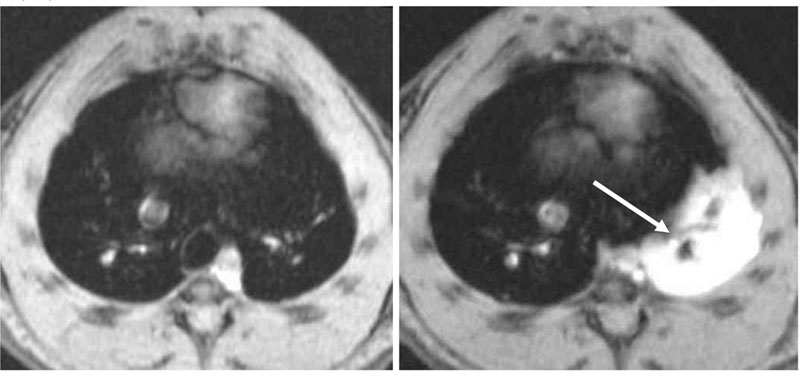
Fig. 2:
Axial images through the chest of an actively sensitized BN rat, acquired before (left) and 24 h after intra-tracheal (i.t.) OVA instillation (right). Note that the lung parenchyma appears dark; however, following allergen, a prominent MRI fluid signal is seen (arrow). This signal is due to edema. The acquisition time for one image is of 1 min.
This correlation provides the basis to address the effects of anti-inflammatory therapies in the allergen model. In one experimental paradigm the drugs are given in a therapeutic regimen 24 h after the challenge with OVA, a time point when an extensive MRI signal is present in the lungs. Treatment with budesonide, a corticosteroid approved for clinical use, accelerates the rate of resolution of the MRI signal (figure 3). The decline in the edematous signal correlates significantly with the reduction in perivascular edema quantified by histology of the lungs. By contrast, BAL fluid markers of inflammation are not affected by budesonide. It seems, accordingly, that the early resolution of MRI edematous signals by the anti-inflammatory drugs does not involve general suppression of the inflammatory response at least as monitored by BAL fluid analysis (5).
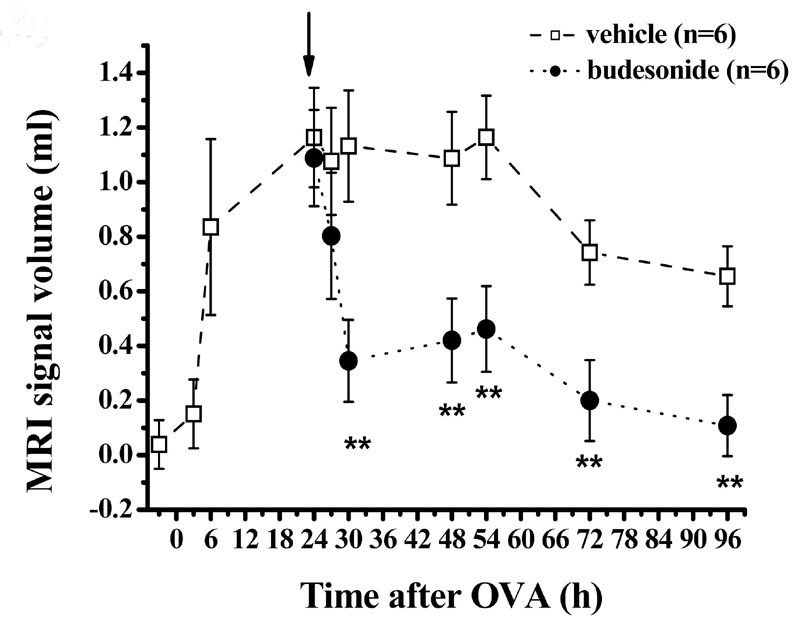
Fig. 3:
Volume of fluid signals detected by MRI in the lungs for an OVA dose of 3 mg/kg (i.t.). Administration of the corticosteroid budesonide (1 mg/kg i.t.) 24 h after OVA (arrow) leads to an acceleration of the resolution of the MRI signals, suggesting a rapid effect of the compound. This effect is not detectable by conventional BAL fluid analysis. Data are expressed as means ± SEM (n=6 rats per group).
Less discomfort, shorter experiments
With MRI one has the potential to shorten the overall experimental duration after injury onset since the technique is able to detect changes before these are reflected in parameters of inflammation present in BAL fluid. This results in less discomfort for the animals.
Less animals and more relevant data
The non-invasive MRI approach results in a significant reduction in the number of animals used for experimentation. Depending on the application, a reduction between 80 to 90% is estimated. Since repeated measurements are feasible, each animal can serve as its own control, thereby reducing the variability of the data. As acquisitions are performed on spontaneously breathing rats, interferences with their well-being and physiological status are minimized. MRI is able to provide data on rapid effects of anti-inflammatory compounds on established inflammation, an information that is not accessible to conventional, post mortem BAL fluid analysis. Thus, data that are more relevant to address therapeutic effects can be obtained using MRI. Anesthesia is the primary limiting factor of the approach. However, following an examination, rats recover from anesthesia within 10-15 minutes.
Overall, MRI provides a global picture of the disease status in the animal model. As this imaging technique is largely available in hospitals, there is potential to address translational aspects from the models in rats to the human situation.
Published updated version of this Bulletin 32/2007 (PDF)
References:
- Beckmann N, Tigani B, Ekatodramis D, Borer R, Mazzoni L, Fozard JR. Pulmonary edema induced by allergen challenge in the rat: noninvasive assessment by magnetic resonance imaging. Magn Reson Med 2001;45:88-95.
- Beckmann N, Tigani B, Sugar R, Jackson AD, Jones G, Mazzoni L, Fozard JR. Noninvasive detection of endotoxin-induced mucus hypersecretion in rat lung by MRI. Am J Physiol Lung Cell Mol Physiol 2002;283:L22-L30.
- Beckmann N, Cannet C, Zurbruegg S, Rudin M, Tigani B. Proton MRI of lung parenchyma reflects allergen-induced airway remodelling and endotoxin-aroused hyperresponsiveness: A step toward ventilation studies in spontaneously breathing rats. Magn Reson Med 2004;52:258-268.
- Karmouty Quintana H, Cannet C, Zurbruegg S, Blé F-X, Fozard JR, Page CP, Beckmann N. Proton MRI as a non-invasive tool to assess elastase-induced lung damage in spontaneously breathing rats. Magn Reson Med 2006; in press.
- Tigani B, Cannet C, Zurbruegg S, Schaeublin E, Mazzoni L, Fozard JR, Beckmann N. Resolution of the oedema associated with allergic pulmonary inflammation in rats assessed noninvasively by magnetic resonance imaging. Br J Pharmacol 2003;140:239-246.
| [*] | Rodent models of airways diseasesTo study specific aspects of human respiratory diseases and its treatment it is necessary to measure the induced symptoms and the impairment of lung function in living animals. For example, key features of asthmatic inflammation (airway hyperresponsiveness, eosinophilic inflammation together with an increase in activated T cells in the airways) are induced in actively sensitized Brown Norway (BN) rats exposed to allergen (ovalbumin, OVA). Alternatively, an inflammation similar to that observed in chronic obstructive pulmonary disease (COPD) patients (neutrophilia and mucus cell metaplasia) can be elicited in rodents by the administration of endotoxin (lipopolysaccharide; LPS). Emphysema, one of the most critical components of COPD, which results in the destruction of lung parenchymal tissue by a number of proteases (neutrophil elastase or matrix metalloproteinases), is induced in rats by the application of a single dose of porcine pancreatic elastase. In all cases the symptoms resemble those seen in patients in the early onset of the disease. The inflammatory status of the lung is routinely inferred from post mortem analyses of broncho-alveolar lavage (BAL) fluid. Occasionally, time consuming histological analysis is also performed. Lung function is assessed in terminal experiments where animals are treated with a muscle relaxant, tracheotomized and artificially ventilated. Airflow, transpulmonary pressure, and airway resistance are determined for each respiratory cycle. Following these measurements animals are killed by an overdose of anesthetic. Clearly, the invasive character of these procedures precludes repeated assessments in the same animal. Therefore the flexibility of MRI was explored in the group of Nicolau Beckmann to obtain anatomical and functional information of the rat lung, with the scope of developing a non-invasive means of analysis. |
| Letzte Änderung: 30.01.2008 |