 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 37
June 2008
Author

PD Dr. Beate Escher’s group has been working on hazard and risk assessment of chemicals and on the development of in-vitro and low complexity assays for water quality assessment for many years. She is working at the Swiss Federal Institute for Aquatic Sciences (Eawag) and teaching at Swiss Federal Institute of Technology (ETH) Zurich. She has been involved in consulting activities and in workgroups on biocaccumulation and is presently chairing the Society of Environmental Toxicology and Chemistry (SETAC) Advisory Group on Bioaccumulation Assessment.
Dr. Jung-Hwan Kwon developed this PDMS-PAMPA system during his Post-doc at Eawag. He is now an assistant professor at Ajou University in South Korea.
Current addresses:
Beate Escher
escher@eawag.ch
Dept. of Environmental Toxicology
Eawag, Swiss Federal Institute of Aquatic Science and Technology
CH-8600 Dübendorf, Switzerland
Jung-Hwan Kwon
jhkwon@ajou.ac.kr
Dept. Environmental Engineering
Ajou University, Wonchun-dong, Yeongtong-gu
Suwon, 443-749, South Korea
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Bioconcentration of chemicals in fish can be assessed in vitro
In this project (No. 100-06), a new in-vitro system was developed for the bioaccumulation assessment of hydrophobic organic environmental pollutants in fish.[*] Twenty pollutants were tested. The measured membrane permeability in the in-vitro assay, called PDMS-PAMPA, was proportional to the passive elimination rate constant in fish. The in-vivo data were very close to predicted values from in-vitro except for metabolized chemicals. For this class of chemicals the PDMS-PAMPA can be coupled to an in-vitro metabolizing system using cellular fractions from fish liver. In addition, a model was developed to predict passive uptake/elimination rate in fish.
Hydrophobic pollutants, not an easy task
For PBT assessment in fish, the focus is on hydrophobic compounds. For these compounds the uptake and elimination by passive diffusion over biological membranes is more relevant than other pathways, such as paracellular diffusion or active transport. Uptake via gill, skin and surface as well as gut uptake are governed by similar passive diffusion processes but there are differences in the permeability barrier and the diffusion gradient.
Passive absorption and elimination through fish gills can be described by the parallel artificial membrane permeability assay (PAMPA)1. But this assay has to be tuned for hydrophobic chemicals, for which resistance of overall permeability is aqueous boundary-layer controlled. In order to overcome the difficulties associated with low aqueous solubility and high membrane affinity, the present modified new system called “PDMS-PAMPA” was developed.
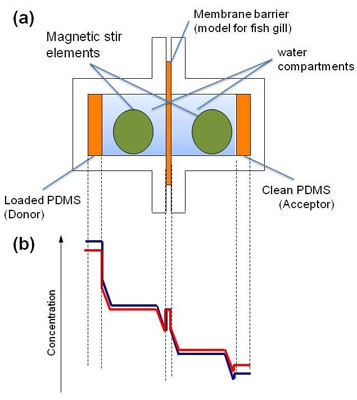
Fig. 1:
1a) Experimental set-up of the PDMS-PAMPA for hydrophobic chemicals.
1b) Typical concentration levels of a test chemical between and in the chambers and membranes at the beginning of the experiment (blue) and a few hours later (red)3.
Silicone is saving lives of fish
In the new assay, silicone disks (polydimethylsiloxane, PDMS) are used as a dosing and sampling system so that no determination of the aqueous concentrations becomes necessary2. The donor PDMS disk preloaded with the test compound is in contact with a water compartment and is cut off from an identical but clean acceptor PDMS disk by a thin PDMS membrane which acts as gill membrane model. (Figure 1). The thickness of the unstirred water layer (UWL) adjacent to all surfaces is adjusted by vigorous tumble stirring to physiological conditions in fish gills. This is a crucial step because for very hydrophobic compounds the rate limiting step of the overall uptake process is actually the slow diffusion through this layer and not the faster diffusion through the gill (model) membrane. Figure 2 shows how the glass cells are assembled and the tumble magnetic stir element is inserted.
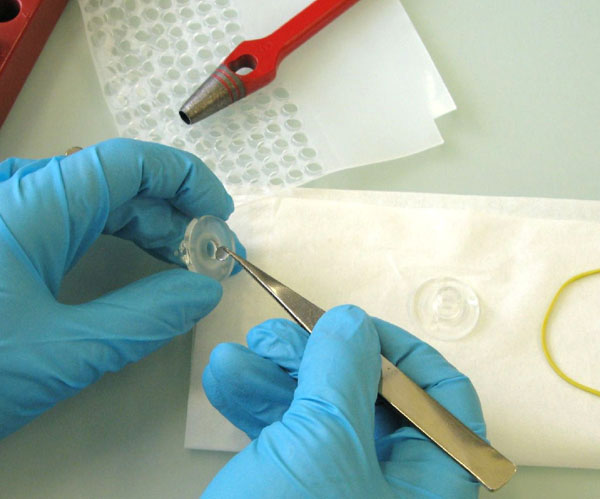
Fig. 2: Preparation of the PDMS-PAMPA system. The acceptor and donor PDMS were cut out from the PDMS sheet in the background (and cleaned prior to use) and inserted into a glass cell. The magnetic tumble stir bar is added before assembling the two half-cells with the thin PDMS gill model membrane in between.
Permeability versus elimination
Then the flux from the donor to the acceptor compartment is measured within 24h. The system does not need to go into steady state, it is sufficient to achieve a measurable change of concentration in the acceptor PDMS disk (see concentration profiles in Figure 1b). For very hydrophobic compounds, it would take too long to wait until the PDMS membrane is saturated. Therefore the membrane was preloaded prior to the experiment with half of the donor PDMS disk concentration (steady state concentration). It was experimentally confirmed that this membrane concentration did not change over the course of the experiment.
Using measured permeability values in PDMS-PAMPA system and measured partition coefficients between PDMS and water3, the artificial in-vitro membrane permeability was normalized with in-vivo elimination rate in small fish3. Figure 3 demonstrates that the measured permeability is proportional to the passive elimination rate constant in fish and can be used to predict the “minimum“ in-vivo elimination rate constant. In the case of additional biochemical interactions between fish tissue and PBT (metabolism or active transport) the in-vivo elimination rate will be modified.
The in-vivo data were very close to predicted PDMS-PAMPA values except for a few polar chemicals (presumably due to differences in hydropgen bonding) and the metabolically active chemicals, such as pyrene and benzo[a]pyrene.
A definitive advantage of the PDMS-PAMPA system lies in the fact that the prediction model to relate the in-vitro results to in-vivo is not just a best-fit model but is a theoretical model based on the underlying mechanistic processes of passive diffusion. Thus, PDMS-PAMPA will be a good in-vitro system for non-metabolizable chemicals.
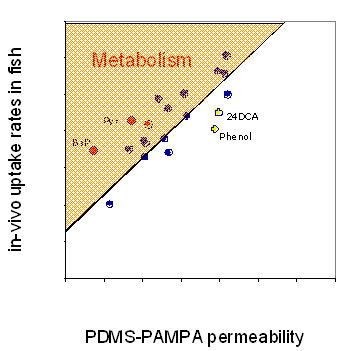
Fig. 3:
Relationship between in-vivo uptake rate constants in fish and PDMS-PAMPA permeability. Outliers are benzo[a]pyrene (BaP) and pyrene (Pyr) due to additional metabolism and compounds with strong H-bonding properties, such as phenol or 2.4-dichloroaniline (24DCA)
Combining diffusion with metabolism
As expected, compounds that are converted in the fish body (liver) into hydrophilic metabolites had a faster in-vivo elimination rate than the one predicted by PDMS-PAMPA3. Therefore it is necessary to combine the in-vitro assay for the passive diffusion of hydrophobic chemicals with in-vitro metabolism assay such as S-9 fractions from fish liver or isolated fish hepatocytes.
Benefits for the 3Rs
Bioconcentration assessment in fish is highly animal and labor intensive. According to the OECD test guideline 305 “Bioconcentration: Flow-through Fish Test“ 4 the test is performed during 28 days in two phases – uptake and depuration – with at least 9 sampling points using at least four fish each. Thus a minimum number of 40 fish is required for the determination of one bioconcentration in fish (BCF) value for one species and one chemical. The proposed in-vitro assay has the potential to for screening assessment and prioritization of necessary in-vivo testing.
Outlook
With the PDMS-PAMPA system there is an in-vitro model for passive uptake available that can tackle compounds of concern, i.e. compounds of high hydrophobicity. The prediction model focuses on the prediction of passive uptake/elimination rate and needs to be further refined and validated with respect to metabolized compounds. After that it can become widely applicable for screening purposes and for deriving input parameters into in-silico models.
PDF version of this Bulletin No. 37
References:
- Kwon, J.-H.; Katz, L. E. Liljestrand, H. M., Use of a parallel artificial membrane system to evaluate passive absorption and elimination in small fish, Environ. Toxicol. Chem./SETAC 2006, 25, 3083-3092.
- Kwon, J.-H.; Wüthrich, T.; Mayer, P.; Escher, B. I., Dynamic permeation method to determine partition coefficients of highly hydrophobic chemicals between poly(dimethylsiloxane) (pdms) and water, Anal. Chem. 2007, 79, 6816-6822.
- Kwon, J.-H.; Escher, B. I., A modified parallel artificial membrane permeability assay for evaluating bioconcentration of highly hydrophobic chemicals in fish, Environ. Sci. Technol. 2008, 42, 1787-1793.
| [*] | Pollutants and BioaccumulationThe implementation of the new European chemicals legislation (REACh) in 2007 led to the safety assessment of a large number of new and existing chemicals. One task is the identification of potential environmental pollutants characterized by a high persistence, bioaccumulation and toxicity (PBT chemicals). This testing includes bioaccumulation assessment in fish and other aquatic species. Bioaccumulation testing is highly animal intensive. Therefore ethical and economical arguments require that in-vitro tools should be developed and used at the screening stage. Bioaccumulation encompasses bioconcentration, i.e. the passive uptake (in fish via the gills), and biomagnification, i.e., the active uptake via ingestion of contaminated food. Bioconcentration integrates the uptake, distribution and elimination of a substance due to water-borne exposure. In fish, bioconcentration is typically dominated by passive uptake processes through the gills due to the high surface area of the gill membranes. Xenobiotic metabolism decreases the bioaccumulation in fish. Thus assessment models and in-vitro methods for fish need to account for passive uptake/elimination and metabolism. For drug development a variety of in-vitro systems are available to estimate the penetration of potential drugs through membranes. Most of these in-vitro assays, such as Caco2 cell lines, the parallel artificial membrane permeability assay (PAMPA) and related assays, can be applied for rather hydrophilic environmental pollutants but require adaptation for hydrophobic PBT chemicals. |
| Letzte Änderung: 20.07.2008 |