 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 52
June 2014
Author

Dr. Olivier Preynat-Seauve directs the research activities of the integrated Medical and Haematological Unit in the Laboratory of Immunohaematology at the University of Geneva. He and his colleagues have developed a culturing system for the in-vitro engineering of diseased human tissues. Using this system, it has been possible to gain an insight into the molecular events that underlie the development of brain tumours, particularly those in which viral activity is suspected of playing a role. In collaboration with other research centres, this group has developed a next-generation-sequencing methodology for the screening of viruses that could be implicated in the growth of glioblastomas. This methodology is currently applied in the screening of blood samples, as well as in the next-generation-sequencing-based analysis of Rhesus genes.
Address:
Dr. Olivier Preynat-Seauve
Olivier.Preynat-Seauve@hcuge.ch
Laboratory of Immunohaematology
Haematology Unit
Geneva University Hospital
Editor
Ernst B. Hunziker, Scientific Adviser of the 3R Research Foundation
A new in-vitro approach to the study of brain tumours: an alternative to in-vivo experiments in animals
Glioblastomas are the most frequent and malignant brain tumours in humans. The prognosis for afflicted patients is poor. With a view to improving our understanding of the biological mechanisms underlying the development of glioblastomas, several types of experimental model have been developed, most of which, however, fail to mimic the pathophysiological microenvironment existing in humans. Experiments in mice call for the introduction of a tumour into the brain, which is a highly invasive procedure for the animal. In Project No. 115-09 the research group of Dr. Olivier Preynat-Seauve has developed and characterized a three-dimensional in-vitro approach to simulate the features of the interaction between tumour cells and nervous tissue (Nayernia et al., 2013). The implementation of this model would help to reduce animal experimentation and facilitate the identification of new therapeutic targets.
Experimental models for the study of brain tumours
Gliomas account for more than 70% of all brain tumours in humans; of these, glioblastomas are the most frequent and malignant histological type. The prognosis for glioblastoma patients is poor: fewer than 3% of the affected individuals are still alive 5 years after the diagnosis. Glioblastomas are characterized by an infiltrative growth of tumour cells into the surrounding healthy brain, thereby leading to the formation of secondary foci. Since the dispersed glioblastoma cells are out of the reach of either surgery, chemotherapy, or irradiation, the tumour cannot be completely destroyed. The invasive activity of the residual cells can thus continue unabated, and this circumstance is believed to account for the poor prognosis.
With a view to improving our understanding of the biological mechanisms underlying the development of glioblastomas, several experimental models have been developed at the cell-line, organ-slice and animal levels. Although significant progress in this field has been made, the vast majority of the existing models fail to mimic the pathophysiological microenvironment of human glioblastomas. Moreover, experiments in mice call for the introduction of a tumour into the brain, which is an extremely invasive procedure having highly deleterious consequences for the animal.
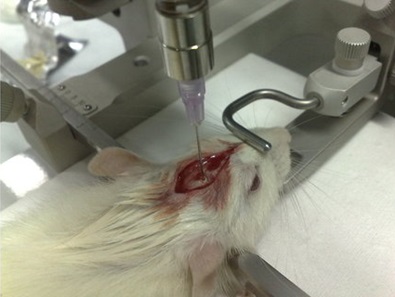
Introduction of tumour cells into a murine brain for the study of a glioblastoma.Although genetically-modified mice develop brain tumours spontaneously, a widely used model for their study involves the direct injection of tumour cells into the brain parenchyma of healthy animals.
The use of human pluripotent stem cells as an alternative to models in animals
We have developed and characterized a three-dimensional in-vitro approach to simulate the features of the interaction between tumour cells and nervous tissue. To achieve this goal, we have implemented a formerly-established air/liquid-interface culturing system, which permits the differentiation of human pluripotent stem cells into nervous tissue (Preynat-Seauve et al., 2009).
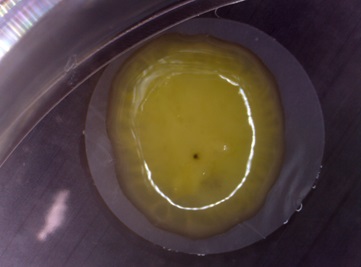
Human nervous tissue generated in vitro from pluripotent stem cells. Pluripotent stem cells were induced to differentiate into neurons using an air/liquid-interface culturing system. The cells are plated on a semi-permeable membrane which favours cell-to-cell contacts and exchanges with air, thus facilitating tissue formation.
By co-culturing glioblastoma cells with the engineered nervous tissue, we have established a human-tissue-sharing situation that resembles the development of glioblastomas in the brain. The histological features include: (i) diffuse invasion characteristics, (ii) secondary foci and (iii) areas of necrosis.
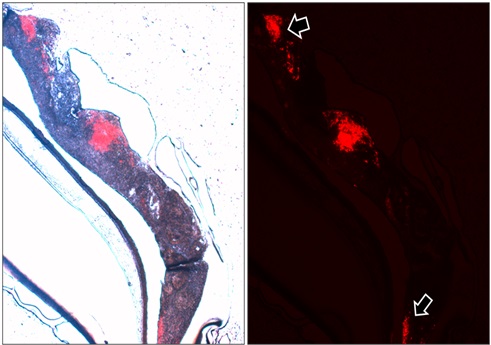
Glioblastoma cells are labeled with a red-fluorescent tag and injected into the engineered nervous tissue. After two weeks, a central mass of tumour cells and distant secondary foci (arrows) were observed. This latter feature is highly specific for glioblastomas.
Using this model, we have demonstrated that general changes in the pattern of gene expression occur when the tumour cells interact with the non-tumoural brain tissue. The in-vitro gene-expression profile bears a close resemblance to the in-vivo situation (predominance of a type-I interferon response).
Our histological and molecular observations reinforce the concept that pluripotent stem cells afford a very attractive approach to the induction of tumours in mice, with the advantage that the interaction between the host and the tumoural tissue can be studied exclusively in the human species. The implementation of this model would help to reduce animal experimentation and facilitate the identification of new therapeutic targets.
PDF version of this Bulletin No. 52
References:
- Preynat-Seauve, O., Suter, D., Tirefort, D. et al.: Development of human nervous tissue upon differentiation of embryonic stem cells in three-dimensional culture. Stem Cells, 27 (3), 509-520, 2009.
- Nayernia, Z., Turchi, L., Cosset, E. et al.: The relationship between brain tumor cell invasion of engineered neural tissues and in-vivo features of glioblastoma. Biomaterials, 34 (33), 8279-8290, 2013.
| Letzte Änderung: 19.11.2014 |