 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 48
February 2012
Authors
Xueya Wang, PhD and Marc Wolf, MD, established the ex vivo mouse aorta perfusion model within the Nanomedicine Research Group of Patrick Hunziker (MD and professor) at the University Hospital Basel, Switzerland. This team develops nanoscale polymer delivery systems for the safe and specific delivery of therapeutic molecules and intelligent materials to target tissues and cells in humans focusing on atherosclerosis and cancer therapy.
Address:
Xueya Wang
wangxu@uhbs.ch
Nanomedicine Group
University Hospital Basel
Petersgraben 4
CH-4031 Basel, Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
A novel ex vivo mouse aorta perfusion model
Atherosclerosis(1)[*] is a major burden of disease worldwide, leading to heart attack, stroke, and sudden death.(2) For many studies investigating vascular pathophysiological processes and drug actions, classical (i.e. in vivo) animal experiments are performed, where one time end point equals one animal (usually mice) to be sacrificed. In project No. 111-08 a novel ex vivo mouse aorta model was developed, allowing for multiple endpoint (i.e. time-lapse) studies with imaging in three spatial dimensions by a confocal laser-scanning microscope.
Studies of vascular pathophysiology and pharmacology
Up to now, to our knowledge, ex vivo mouse artery models exist only for peripheral arteries such as the carotid. However, in classical atherosclerosis research utilizing the in vivo approach in experimental animals, the aorta commonly is the only artery analyzed.(3) As the pathobiology of atherosclerosis is dependent on the location within the arterial tree,(4) an ex vivo model of the mouse aorta is highly relevant as – due to pathobiologically similar cellular substrate – it would allow comparability with data from classical in vivo experiments with the ex vivo generated data. Also, the aorta exhibits multiple atherosclerotic plaques, whereas the carotid bifurcation normally has one plaque only. Furthermore, the possibility to control the composition of the perfusate reduces experimental variability as encountered in vivo due to variations induced by animal weight, blood volume, and systemic clearance.
Preparation of the Aorta
ApoE -/- mice, a frequently used transgenic mouse model of atherosclerosis, were fed a fat-rich diet in order to accelerate the development of atherosclerotic lesions. A mouse was sacrificed and the vessel system rinsed with buffer. The aorta was cut proximally to the aortic bifurcation and the heart basis was cut from the rest of the heart. Micro-catheters were inserted from proximal and distal apertures into the aorta and ligated with microsurgical thread. The aorta was then harvested, freed from surrounding perivascular fat and embedded in liquid PDMS (poly-dimethyl-siloxane) on a microscope glass slide, and stored at 37°C for two hours, allowing polymerization of the PDMS to a transparent block. The embedded aorta was then connected to the perfusion system consisting of a peristaltic pump to mimic the physiological perfusion, a warming plate for the pre-warming of the medium, a syringe pump for the supply of fresh medium and a medium drain (Figure 1). The PDMS-embedded aorta was placed in the stage-top incubator of the confocal microscope at 37°C.
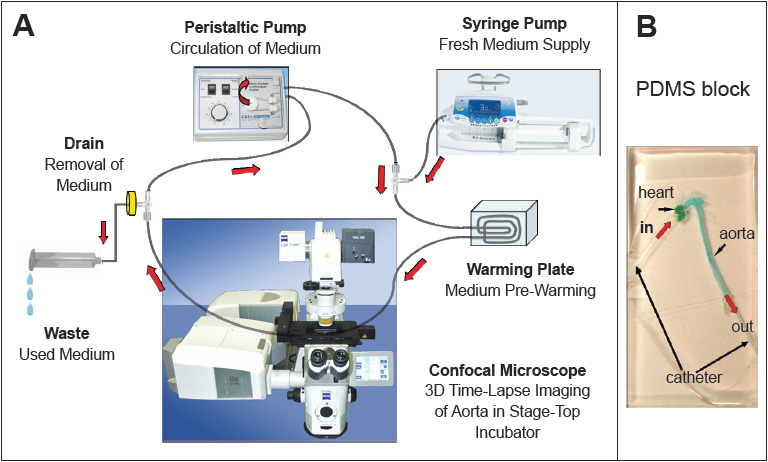
Fig 1
(A) The circulation system consisting of the peristaltic pump, the confocal microscope, and
(B) the PDMS embedded aorta, mounted into the microscope incubation chamber.
Viability of the aortic tissue after perfusion
In order to assess viability of the aortic tissue after 24 hours of perfusion, the aorta was stained with HE (to visualize the histological structure), Verhoeff van-Gieson stain (to confirm integrity of elastin and collagen fibers), VCAM-1 antibody (to confirm integrity of the endothelium), as well as caspase-3 (to screen for cellular apoptosis). All the stainings showed identical results compared to the control aorta assessed immediately after animal sacrifice. This confirms the viability of the aortic tissue after 24 hours of perfusion, and therefore the suitability of the model for biological experiments.
Targeting of atherosclerotic plaques
In order to test the capability of the perfusion system to interrogate biological parameters of the vessel wall, we added a Cy5 labeled aptamer to the circulating cell culture medium. The aptamer binds to specific macrophage cell-surface receptors. Macrophages are crucial for the development of atherosclerotic plaques, and can be found inside plaques. The addition of the aptamer should therefore allow the staining of the plaques, more specifically of the intra-plaque macrophages. The resulting data from the 20 hour time-lapse experiment are presented in Figure 2. The time-plot of the intra-plaque fluorescence signal exhibits pharmacokinetically realistic behavior, with saturation curve-like behavior during the two hours of incubation, and a constant signal during the wash-out procedure (remaining 18 hours) indicating stable binding to the target. In order to verify the co-localization of the observed signal with intra-plaque macrophages, tissue slices of the aorta used for the perfusion experiment were prepared. The slices were stained with Bodipy (stains intra-plaque lipid droplets) and with a CD45 (leukocyte cell surface protein) antibody. The pictures show complete co-localization of the Bodipy and Cy5 signals, and partial co-localization of the CD45 and Cy5 signals. The latter relates to the known fact that macrophage distribution in plaques can be highly heterogeneous.(5) In summary, these results prove the feasibility of this novel ex vivo model of the atherosclerotic mouse aorta for studying pharmaceutical targeting at multiple time points using a single mouse tissue for perfusate-mediated time-lapse studies.
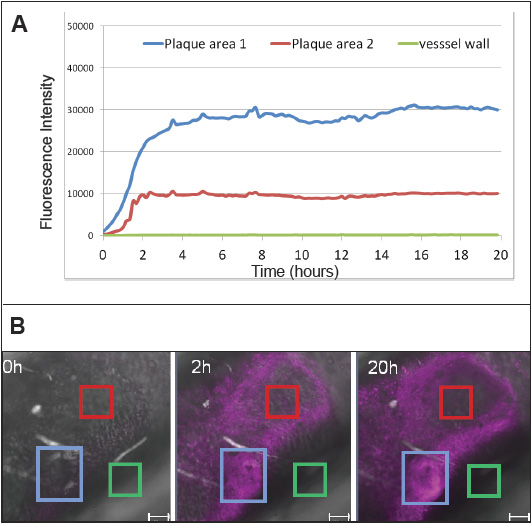
Fig 2
(A) Time-lapse experiment with perfusion of the aorta for 20 hours. During the first two hours, a fluorescently labeled aptamer was included in the medium. At two hours, the medium was exchanged; the washout period lasted for the remaining 18 hours. The mean intensity of three regions of interest of a focal slice from confocal imaging was plotted over time.
(B) The fluorescence intensity in one position (magenta) at different time points at three regions of interest in the image data generated from one experiment. The blue rectangle corresponds to “plaque area 1” on the time-line graph, the red one to “plaque area 2” and the green to a plaque-free vessel wall area (green line in time-line graph) and serves as a negative control. The scale bar is 100μm.
Impact on the 3Rs
This novel approach can reduce the number of animal experiments and refines the data acquired from a single animal, due to multiple end-point generation, direct comparability with experimental in vivo data, increased statistical power due to multiple lesions in a single sample, and reduced variability of experimental parameters. Also, experiments are performed ex vivo in the living aorta after sacrificing the animal, thus eliminating pain and suffering in the experimental protocol as encountered in in vivo experiments.
Aptamer: oligonucleic acid or peptide molecules that bind to a specific target molecule
VCAM-1: vascular cell adhesion molecule 1
Cy5: water-soluble fluorescent dye of the cyanine dye family.
HE: haematoxylin-eosin
PDF version of this Bulletin No. 48
References:
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. 2005. N Engl J Med. 352(16):1685-95.
- Lopez, A.D., C.D. Mathers, M. Ezzati, D.T. Jamison, C.H.L. Murray. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 367:1747-57.
- Whitman, S.C. A practical approach to using mice in atherosclerosis research. 2004. Clin Biochem Rev. 25:81-93.
- VanderLaan, P.A., C.A. Reardon, G.S. Getz. Site specificity of atherosclerosis. 2004. Arterioscler Thromb Vasc Biol. 24:12-22.
- Johnson, J.L., A.C. Newby. 2009. Macrophage heterogeneity in atherosclerotic plaques. Current Opinion in Lipidology. 20:370-78.
- Wang X, Wolf MP, Bänziger Keel R, Lehner R, and Hunziker PR. 2012. Polydimethylsiloxane embedded mouse aorta ex vivo perfusion model: proof-of-concept study focusing on atherosclerosis, Journal of Biomedical Optics 17(7), doi:10.1117/1.JBO.17.7.076006.
| [*] | AtherosclerosisAtherosclerosis is one of the leading causes of death worldwide(2) and consumes billions of dollars in health care. Organs such as the heart (heart attack), brain (stroke), aorta (aortic rupture), kidney failure and legs (gangrene) can be affected. Atherosclerosis has an important vascular inflammation aspect relevant for plaque development and plaque rupture (vulnerable plaque). First changes of the arterial vessel walls can be observed already in healthy teenagers (so-called fatty streaks, corresponding to sub-endothelial lipid accumulations). The progression of these early lesions can lead to partial occlusion of arteries, thus reducing their maximum flow rate. This can lead to symptoms such as angina pectoris, cardiac arrhythmia, syncope, renal insufficiency and leg claudication. The major complication risk in advanced atherosclerotic lesions is sudden plaque rupture or erosion of the endothelial cell layer with subsequent thrombosis, complete occlusion of the vessel potentially leading to distal embolism in more peripheral arterial segments. Clinically, this manifests as acute myocardial infarction, stroke, and necrosis of other organs. On a molecular and cellular level, the disease starts with blood-flow dependant changes of the luminal endothelial cell surface at characteristic lesion locations. Lipid particles (low-density lipoprotein, abbreviated LDL) from the blood then extravasate to the sub-endothelial space. This leads to further activation of endothelial cells and transmigration of monocytes from the blood. The monocytes differentiate into macrophages that take up (i.e. engulf) the oxidized lipid particles by receptor-mediated endocytosis. A multitude of secreted locally acting (i.e. paracrine) cytokines lead to migration and proliferation of vascular smooth muscle cells to the sub-endothelial space, where they form a fibrous cap. The macrophages can undergo apoptosis or necrosis due to lipid particle overload, thus forming a lipid necrotic core. Newly formed blood vessels spread into the plaque tissue (neo-vascularization), leading to intra-lesional hemorrhage. Finally, the fibrous cap can rupture, or the endothelial layer above the cap be eroded. This leads to the exposure of a thrombogenic surface to the blood and the rapid formation of a blood clot (thrombus). |
| Letzte Änderung: 30.01.2013 |