 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 45
February 2011
Author
Prof. Dr. Paul Honegger and his group at the University of Lausanne developed aggregating cultures of mechanically dissociated primary fetal brain cells which provide an excellent system for neurobiological studies of cellular growth and differentiation. In his present investigation he used this serum-free aggregating brain cell cultures as a reference culture system.
Address:
Paul Honegger
paul.honegger@unil.ch
Département de Physiologie
Université de Lausanne
Rue du Bugnon 7
CH-1005 Lausanne
Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Serum-free defined media, a largely unsolved problem in cell culture
Cell culture methods often lead to the reduction of animals used for biological research and industrial applications. Most of these cell culture systems rely on the use of fetal bovine serum (FBS), a supplement of undefined and variable composition.[*] In the present project (No 109-08) the utility of blood serum or plasma proteins for FBS replacement was investigated. Unexpectedly it was found, that the beneficial effects of serum do not reside in any of the examined serum lipoprotein fractions (VLDL/IDL, LDL, HDL), but remain in the lipoprotein-deficient macromolecular FBS fraction.
How to find reproducible culture conditions?
To avoid the use of serum in culture media, two approaches are currently being employed.(1) One is to replace serum by other biological but as yet inadequately defined mixtures such as extracts from plants, animal tissues or cells. Another is to adapt useful cell lines individually for survival and growth in serum-free culture media. The latter limits the choice of cell culture models, possibly excluding cultures of normal, untransformed cells, which are the most interesting and versatile for basic research. The further identification of serum components that are obviously required by cultured cells is of less high priority. It appears reasonable to assume that these particular serum components would be required by all cell types. The ultimate goal remains to find a chemically defined culture medium appropriate for a broad range of cell culture systems. Such a medium should be free of undefined chemicals and mixtures from animal-derived components, and its exact composition should be published in papers and recorded in databases.
Are lipids essential for cell culture?
Of all the components taken into account for cultured cells, the lipids are the most neglected ones. Both linoleic and linolenic acid are essential fatty acids but are often absent from culture media. Moreover, many cell lines would need additional fatty acid derivatives, since they often do not express a normal cell's entire set of metabolic enzymes (e.g., lack of elongases) for the biosynthesis of essential metabolites.
Cholesterol is another component under discussion. It is an important building block of all cell membranes, and an important metabolite in many biosynthetic pathways. Since cholesterol is practically insoluble in aqueous solutions, it is generally absent from culture media. Although most of the cells can theoretically synthesize cholesterol, the biosynthesis is slow and energy-consuming. Therefore, the amount of cholesterol available in vitro may be insufficient, particularly during cell proliferation, repair and maintenance of organ-specific functions (e.g. in cells of the nervous system during neurite extension, synaptogenesis, and myelination).
Role of lipoproteins
In the organism, liposoluble nutrients such as cholesterol, fatty acids and lipophilic vitamins reach the cells by means of specific apolipoproteins which form complexes with the lipids, the so-called lipoproteins (LPs), and which recognize specific membrane receptors expressed by the cells. The LPs in blood serum or plasma can be isolated by sodium-bromide (NaBr)-density centrifugation. The conventional 3 fractions contain very low density/intermediate density, low density, and high density lipoproteins (VLDL/IDL, LDL, HDL), respectively. Knowing that lipids, and particularly cholesterol, are absent from most conventional culture media, the working hypothesis of the present investigation was that the LPs represented the still undefined serum component(s) required for cell culture.
Reference cell culture
Aggregating brain cell cultures were used as a model for LP testing. This 3D primary cell culture system prepared from embryonic rat brain cells (2-5) contains all types of brain cells (i.e., neurons, astrocytes, oligodendrocytes, and microglia) that are enabled to grow and mature in serum-free medium, and to form highly differentiated structures such as myelinated axons, synapses, and functional neuronal networks. Previous work showed that this culture system was ideal for the study of hormones, growth factors and other signaling molecules on cellular growth, maturation and homeostasis.
Effect of LP fractions compared
Lipoprotein (LP) fractions (VLDL/IDL, LDL, HDL) were isolated by NaBr-density centrifugation from (i) fetal bovine serum, (ii) newborn bovine serum, and (iii) human plasma. Each fraction was dialyzed before use to remove NaBr, and different serum controls, i.e., FBS, dialyzed FBS, and LP-free FBS, were heat-inactivated (30 min, 56°C), a standard procedure used for complement inactivation. The 3 LP fractions together with the LP-free serum and the whole sera as controls were tested in aggregating brain cell cultures. The maturation was monitored using 4 cell type-specific enzymatic biomarkers,2,3 i.e., 2',3'-cyclic nucleotide 3'-phosphohydrolase (CNPase, oligodendrocyte-specific); glutamine synthetase (GS, astrocyte-specific) as well as choline acetyltransferase (ChAT) and glutamate decarboxylase (GAD), both neuron-specific.
LPs not critical for cell culture
Table 1 shows a representative sample of the data obtained. Combined results from all experiments lead to the conclusion that none of the three isolated LP fractions (VLDL/IDL, LDL, HDL) display FBS maturation-enhancing activity. Instead, this activity appears in the last LP-deficient fraction, indicating that the isolated LP fractions are unable to replace FBS in cell culture. Similar negative results were obtained with the LP fraction of newborn bovine serum (NBS) and of human plasma, from which it was possible to isolate higher amounts of LP. Negative results were also obtained using several purified blood proteins, including fibronectin, alpha1-antitrypsin, fibrinogen, and tissue-type plasminogen activator. In terms of the 3R philosophy, it is noteworthy that NBS was almost as stimulatory as FBS. NBS may therefore come to replace FBS.
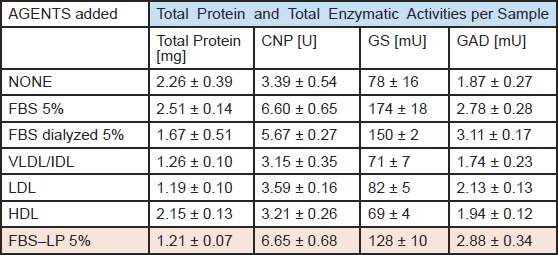
Table 1
Effect of bovine lipoprotein fractions prepared from FBS, and of lipid-deficient FBS, in comparison to FBS, on neuronal and glial maturation in aggregating brain cell cultures.
The data are mean values ± SD of 3 - 4 replicate cultures.
Detailed results and conditions used for dialysis, lipid fractionation, heat inactivation, amount of lipoproteins added and additional enzymatic activities are given at http://www.forschung3r.ch/de/projects/pr_109_08.html.
Characteristics of LP-deficient factor(s)
The finding that it is the LP-free fraction that displays beneficial serum “activity” despite dialysis and heating to 56o C indicates that the component(s) of interest are macromolecules or macromolecular complexes; small molecules such as hormones or cytokines can be excluded. In principle, it should be possible to isolate, separate, and individually test these macromolecules for their bioactivity using a reference culture. Once identified, the macromolecules or their active parts might be obtained synthetically, which would open the way to cell culture work with higher reproducibility, and to reduced suffering of unborn calves.
PDF version of this Bulletin No. 45
References:
- van der Valk J., Brunner D., De Smet K., Svenningsen Å.F., Honegger P., Knudsen L.E., Lindl T., Noraberg J., Price A., Scarino M.L. and Gstraunthaler G. Optimization of chemically defined cell culture media – replacing fetal bovine serum in mammalian in vitro methods. Toxicology in Vitro 24 (2010) 1053-1063.
- Honegger P., Lenoir D., Favrod P. Growth and differentiation of aggregating fetal brain cells in a serum-free defined medium. Nature 282 (1979) 305-308.
- Honegger P., Monnet-Tschudi F. Aggregating neural cell cultures. In S. Fedoroff and A. Richardson (eds.) Protocols for Neural Cell Culture, 3rd ed., Humana Press, Totowa, N.J. (2001), pp. 199-218.
- Zurich M.-G., Lengacher S., Braissant O., Monnet-Tschudi F., Pellerin L., Honegger P. Unusual atrocyte reactivity caused by the food mycotoxin ochratoxin A in aggregating rat brain cell cultures. Neuroscience 134 (2005) 771-782.
- Honegger P. and Zurich M.-G. Preparation and use of serum-free aggregating brain cell cultures for routine neurotoxicity screening. in M. Aschner et al. (eds), Cell Culture Techniques, Neuromethods, vol. 56 (2011) Springer Science+Business Media.
| [*] | Fetal bovine serum and the 3RsIn the past, great efforts have been made to identify the chemical conditions necessary for the in-vitro survival, growth and maintenance of cells.(1,2) The identification of essential components, including nutrients, vitamins, trace elements, hormones, protease inhibitors, cytokines and attachment factors provided the groundwork for the preparation and use of chemically defined culture media. Nevertheless, serum-free/chemically defined cell culture media are still rarely used for mammalian cells. Most standard cell culture protocols require supplementation with fetal animal serum, typically 10% v/v FBS. This causes several serious problems, two of which conflict with the 3R rules, insofar as FBS collection inflicts suffering on unborn calves, and complex, variable, incompletely defined FBS composition leads to irreproducible cell culture work. Seasonal and regional variations in serum composition, resulting in batch-to-batch variations, further aggravate the situation. Moreover, there is a likelihood of contamination with BSE or viruses, contraindicating the use of FBS for the preparation of biological and pharmacological products. |
| Letzte Änderung: 06.10.2011 |