 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 53
November 2014
Authors

Professor Thiel holds the Chair in Veterinary Virology at the University of Bern. His research interests focus on the biology of coronaviruses and on important virus-host interactions that impact innate immune responses and viral pathogenicity. Dr. Ronald Dijkman joined Prof. Thiel’s laboratory in 2011 and introduced a system for the culturing of primary human airway epithelial cells that proved to be of great value in studying the early events of coronavirus infections in the natural target cells of the respiratory tract. Hulda R. Jonsdottir, who is engaged on this study as a PhD-student, gained ample experience with similar primary airway culturing systems during her Master’s project. The team has established a solid basis for improving this in-vitro cell-culture model, also with a view to reducing and replacing experiments in living animals.
Address:
Prof. Volker Thiel
volker.thiel@vetsuisse.unibe.ch
Dr. Ronald Dijkman
ronald.dijkman@vetsuisse.unibe.ch
Hulda R. Jonsdottir
hulda.jonsdottir@vetsuisse.unibe.ch
Institute of Virology and Immunology, University of Bern
CH-3012 Bern
Switzerland
Editor
Ernst B. Hunziker, Scientific Adviser of the 3R Research Foundation
Genetic manipulation of the human airway epithelium – a paradigmatic system to study host responses to human respiratory viruses
The airway epithelium is the main port of entry for many respiratory pathogens and an important barrier to infection. This barrier must be penetrated by respiratory viruses if they are to establish a robust infection, which is necessary for their propagation within a host and for their transmission to new ones. Airway epithelial cells are believed to possess a number of anti-viral mechanisms that not only prevent infection with, but also limit the spread and transmission of viruses. Experimental systems that are suitable for studying the mechanistic details of these defence strategies are scarce. Specifically, the identification and detailed characterization of anti-viral functions at the molecular level call for an experimental system that is versatile, flexible and biologically relevant. The project (128-11) that was supported by the 3R Research Foundation Switzerland aimed to establish a unique in-vitro airway epithelial-cell culturing system that is amenable to genetic manipulation.
Preferential use of animal models in the study of respiratory viruses
The analysis of basic virus-host interactions at the molecular level is still preferentially performed in animal models. This is particularly true for human respiratory-virus infections, for which a murine model is available. The main reason for this preference is that a murine model of viral infection permits the use of widely available transgenic or knock-out strains of mice. Consequently, there is even the tendency to adapt human respiratory viruses for replication in mice with the view of generating a model that will recapitulate the disease in humans.
Primary airway epithelial-cell cultures as an alternative to animal models
Recent advances in the cultivation of primary human airway epithelia (HAE) have permitted the study of human respiratory viral infections in a culturing system that morphologically and functionally resembles human airways in vivo. The HAE culturing system thus holds the potential to replace many animal experiments. HAE-cultures are based on primary human bronchial epithelial cells, which are obtained from tissue of the upper airways. Following their isolation, the airway epithelial cells can be cultured for about 2-3 passages in vitro, which allows for a moderate increase in cell numbers. The cells are then induced to differentiate into a pseudostratified HAE-culture by manipulation with a chemically-defined growth medium. During this process, the cells are grown on inserts, which are placed within the culture wells. The cells are supplied with medium, nutrients and specific growth factors from the basolateral side, whilst the apical side is exposed to air. These so-called “air-liquid-interface” (ALI) cultures of fully-differentiated cells form a pseudostratified epithelial layer that (i) contains basal, secretory, columnar and ciliated cell populations, and (ii) generates mucus (1).
Genetic manipulation of primary airway epithelial cultures – major hurdles surmounted
Although the HAE culturing system recapitulates many of the facets that are characteristic of the human airways in vivo, it suffers from the major limitation of not being (as yet) amenable to genetic manipulation. As in the case of the murine system, it would be advantageous to establish experimental systems for primary human cells with specific gene-deficiencies or trans-gene expression.
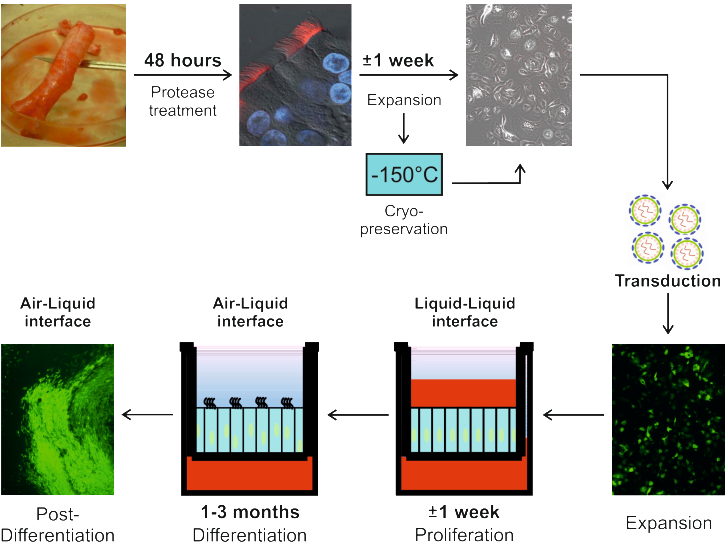
Fig 1.: Schematic representation of the generation of genetically-manipulated primary HAE-cultures.
We therefore evaluated the possibility of using lentiviral vectors to introduce short hairpin RNAs (shRNAs; to knock-down specific mRNAs) or genes of interest (for trans-gene expression). Major hurdles that were encountered in achieving this goal included (i) insufficient transduction efficacy of the fully-differentiated HAE-cultures, (ii) potential interference of lentiviral transduction with the cellular composition of fully-differentiated HAE-cultures, and (iii) limited potential for the propagation of primary airway cells.
These major hurdles were surmounted during the course of the project. The best transduction efficacies were achieved when the epithelial cells were still in an undifferentiated state and transduced in suspension (Figure 1).
Importantly, we observed that the transduced cells (i.e., those expressing the transgene) were capable of undergoing full differentiation into a pseudostratified epithelial layer and that all cell types comprising the epithelium remained unchanged (Figure 2).
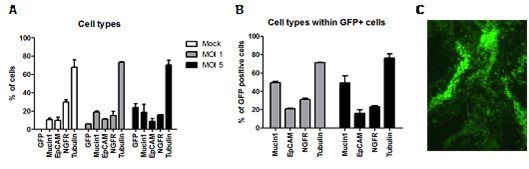
Fig. 2: Analysis of transduced and fully-differentiated HAE-cultures. The cellular composition of the HAE-cultures was assessed by flow cytometry (A,B), and the transgene expression (GFP) of the transduced and fully-differentiated HAE-cultures by fluorescence microscopy (C).
To obtain epithelial cultures that contained (almost) exclusively transduced cells, a selection step was introduced, which was based on the cell-surface expression of the Cherry-Picker protein (Figure 3).
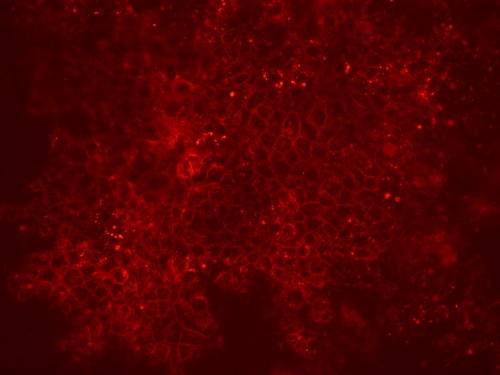
Fig. 3:Cherry-Picker protein expression on the surface of lentivirus-transduced cells.
Cells expressing this protein can be bound to specific antibodies that are coupled to magnetic beads, thereby permitting magnetic cell-sorting. Since several passages are necessary for this process, it was very important to find a way of overcoming the 3-passage limit for the culturing of primary airway cells. This problem was resolved by employing the Rho-kinase inhibitor Y-27632, which has been described to prolong the basal cell phenotype of primary epithelial cells in culture. Most importantly, Y-27632-treated cells were still capable of fully differentiating into pseudostratified epithelial layers during late passages, whereas non-treated cells failed to form fully-differentiated layers after passage 3 (Figure 4). This approach represents a significant improvement, since it permits without passage or time restrictions an assessment of the expression of the selection (e.g., the Cherry-Picker) gene, the selection of transduced cells, and even an extension of the period of proliferation of the transduced cells to obtain larger numbers (e.g., for storage).
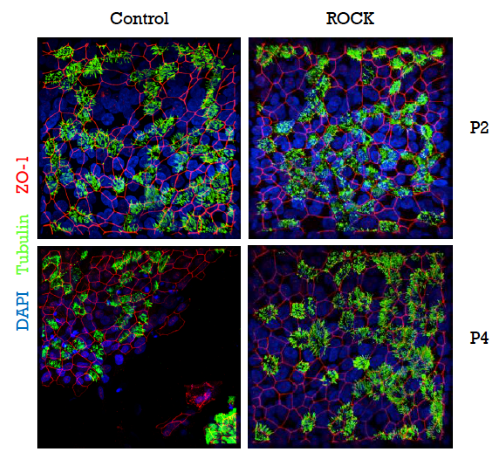
Fig. 4: Rho kinase inhibitor Y-27632-treated cells retain the capacity to form intact and fully-differentiated HAE-cultures after passage 3. Tight junctions, red (ZO-1); cilia, green (tubulin); nuclei, blue (DAPI).
Expect the unexpected – The MERS-coronavirus outbreak
During the course of this project, we witnessed the emergence of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). MERS-CoV is associated with severe respiratory disease and has an exceptionally high case fatality of approximately 40%. Initially, it was not known either how efficiently the MERS-CoV could replicate on the human airway epithelium or whether any measures were available to inhibit MERS-CoV replication in the human airway epithelium, indeed, even the nature of the target cell(s) was unknown. Since primary HAE-cultures were immediately at our command, we could participate in a number of international studies. We were the first to demonstrate that MERS-CoV can efficiently replicate on the primary human epithelium, which highlights its zoonotic potential (2).
Importantly, the treatment of MERS-CoV-infected HAE-cultures with interferon-alpha and interferon-lambda significantly reduced viral replication. Hence, this strategy opens up a therapeutic option for MERS-CoV patients (2).
In another study, we demonstrated that the cellular receptor for MERS-CoV dipeptidyl peptidase 4 (DPP4) is expressed on non-ciliated cells within human bronchial pulmonary tissue and in primary HAE-cultures (3). The discovery of the MERS-CoV receptor offered the basis for numerous further studies.
These investigations have revealed some species to be susceptible to MERS-CoV infection (those with DPP4 that is highly homologous to that in humans), and others to be only barely or not at all susceptible to MERS-CoV infection (those with DPP4 that has only limited homology to that in humans). Finally, we demonstrated that a novel type of coronavirus inhibitor, which targets membrane-bound RNA-synthesis, can efficiently inhibit MERS-CoV replication in HAE-cultures (4).
Relevance for 3R
The generation of a primary airway epithelial-cell culturing system that is amenable to genetic manipulation is near completion. The availability of an HAE culturing system that permits the inactivation of particular genes or the expression of particular genes of interest in trans would permit the replacement of many animal experiments that are based on the use of specific transgenic and knock-out strains of mice. Moreover, by using the protocol that we have established for the lentivirus-based genetic manipulation of human epithelial cells, it would be possible to genetically modify the primary epithelial cells of animal species, and we anticipate that this system will serve as a paradigm for the molecular analysis of host-pathogen interactions at the port of entry of many respiratory pathogens.
PDF version of this Bulletin No. 53
References:
- H.R. Jonsdottir and R. Dijkman. Characterization of human coronaviruses on well-differentiated human airway epithelial cell cultures. In “Coronaviruses: Methods and Protocols”, edited by H.J. Maier, E. Bickerton, and P. Britton. Springer, submitted.
- Kindler, E., Jonsdottir, H., Muth, D., Hamming, O., Hartmann, R., Rodriguez, R., Geffers, R., Fouchier, R.A.M., Drosten, C., Müller, M.A., Dijkman, R., and Thiel, V. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. 2013. mBio 4(1): doi:10.1128/mBio.00611-12.
- Stalin Raj, V., Mou, H., Smits, S.L., Dekkers, D.H.W., Müller, M.A., Dijkman, R., Muth, D., Demmers, J.A.A., Zaki, A., Fouchier, R.A.M., Thiel, V., Drosten, C., Rottier, P.J.M., Osterhaus, A.D.M.E., Bosch, B.J., and Haagmans, B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. 2013. Nature 495:251-254.
- Lundin A., Dijkman R., Bergström T., Kann N., Adamiak B., Hannoun C., Kindler E., Jónsdóttir H.R., Muth D., Kint J., Forlenza M., Müller M.A., Drosten C., Thiel V, and E. Trybala. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. 2014. PLoS Pathog.10(5):e1004166.
| Letzte Änderung: 19.11.2014 |