|
 | On 13 November 2018 the Federal Foundations Supervisory Authority announced that "The 3R Research Foundation (Reduce, Refine and Replace Animal Experimentation) would be put into liquidation and dissolved."
Once the liquidation process has been completed, the Supervisory Authority will issue an order to the Berne Cantonal Commercial Register Office to "delete the 3R Research Foundation (Reduce, Refine and Replace Animal Experimentation) in liquidation" from the commercial register. The dissolution of the Foundation brings to an end 30 years of activity during which it was able to fund 146 research projects with a total amount of CHF 18,787,008.33.
|
|
|
 | Validation of a novel cell-based approach to studying thyroidal physiology: Reduction and/or replacement of experiments with rodents.
Dr. Gerasimos Sykiotis, Endocrinology, Diabetology and Metabolism Department, University Hospital of the Canton of Vaud, Lausanne The majority of studies of the various mechanisms that cause diseases of the thyroid involve the use of mice and rats. The aim of this project was to develop a thyroid cell system (follicle) in vitro which would enable the necessary experiments to be carried out without the use of laboratory animals. As functioning units, these cell systems (follicles) are characterised by a complex structure and are subject to complicated control mechanisms.
In collaboration with scientists from other countries, the research team succeeded in culturing such active cell groups in vitro and demonstrating that the latter could indeed produce functioning thyroid hormones. This now opens the way for further investigations concerning thyroid disease in vitro without the need to use laboratory animals, or at least to involve a far smaller number.
Project 146-15 |
|
|
 | Development of in vitro 3D multi-cellular culture models to study the role of heterotypic cellular interactions in colorectal cancer invasion.
Prof. Curzio Rüegg, Faculty of Science and Medicine, University of Fribourg At present the focus of research into the biology of colorectal cancer is on the interaction of the cancer cells with the body's normal cells such as blood vessel cells, connective tissue cells, immune defence cells, etc. These interactions are an essential part of the mechanism by which the disease spreads. It is difficult to create an experimental simulation of these complex cellular interactions in vitro, which is why laboratory animals are usually used in this type of research.
In order to reduce or even replace animal experimentation, the research team is proposing a new, 3-dimensional in vitro approach (organ-like model) which should make it possible to carry out more detailed analyses in vitro of the intercellular mechanisms involved.
The team have been able to obtain and validate the first promising data concerning blood vessel cells and connective tissue cells as well as creating an organ-like model in vitro. This new in vitro model has shown that it may well be used in the future for quantifying the risk of metastases in vivo.
Project 144-15 |
|
|
 | An advanced in vitro model of pulmonary inflammation based on novel lung-on-a-chip technology
Prof. Olivier Guenat, ARTORG Center, Lung Regeneration Tech, University of Berne Various types of lung disease, forms of pulmonary inflammation and associated lung oedema are frequently seen in human medicine and often prove to be terminal. As a rule, experiments aimed at investigating the pathophysiology behind these diseases and testing of new treatments involve severe suffering for laboratory animals.
The aim of this project was to develop a new in vitro system whereby the pathology of these diseases could be simulated by using "lung-on-a-chip" technology. The inclusion of the relevant inflammation parameters increases the complexity of this multi-cellular in vitro system. The authors of this project have succeeded in achieving their basic aims. It was also possible to include the inflammatory aspects, which play an important role in the pathology, in the lung-on-a-chip system. This makes the system especially relevant for further in vitro experimentation in this field.
Project 143-15 |
|
|
 | Development of an in vitro potency assay for the Clostridium chauvoei vaccine: Replacement of the guinea pig challenge potency test
Prof. Joachim Frey, Institute of Veterinary Bacteriology, University of Berne Black leg disease is an acute febrile illness seen in cattle and sheep that is caused by the Clostridium chauvoei bacterium. The disease causes extreme suffering and quickly leads to the death of the animals involved. Owing to the acute lethal progress of the disease, treatments are almost always ineffective. Protecting the livestock through vaccination would seem to be the most effective preventive measure. Guinea pigs are used to test the potential effectiveness of vaccines and in this process the guinea pigs are subjected to severe stress and pain.
The aim of this project was to develop a reliable in vitro test to replace the in vivo testing in guinea pigs. Prof. Frey and his team have succeeded in developing such an in vitro test which will render the use of laboratory animals unnecessary.
Project 136-13 |
|
|
| Combining computational modelling with in vitro cellular responses in order to predict chemical impact on fish growth
Prof. Kristin Schirmer, EAWAG, Dübendorf Each year, hundreds of thousands of fish are used in legally required experiments to test for possible adverse side-effects of chemicals on the biologial growth of organisms or to identify any potential poisonous impact of new chemical substances intended for use in industry and in the home.
In this project a new computational model was developed for carrying out the corresponding tests in the future through calculations using a comprehensive database containing toxicity test data obtained in vitro from such fish. Furthermore, researchers should also be able to use the model to predict possible inhibitory effects on the growth of fish embryos, an established parameter for potential environmental damage.
Project 145-15 |
|
|
 | Validation of human stem-cell pluripotency using a bioreactor-based culturing system instead of a murine model to effect the development of embryoid bodies into teratomas
Prof. Christian de Geyter, Department of Biomedicine, University Hospital, Basle After stem cells from donor tissue have been isolated (or cultured) it is necessary to test whether these cells still retain the ability, which is typical of stem cells, to differentiate into various types of tissue (pluripotency). According to international guidelines, such testing for pluripotency of stem cells is normally carried out using mice whose immune system is non-functional.
Prof. De Geyter's research team have succeeded in creating a three-dimensional, bioreactor-based culturing system whereby stem cell candidates can be tested for the ability to actually produce the three germ layers.
Project 142-14 |
|
|
 | In-vitro engineering of a human cell-based three-dimensional dynamic model of atherosclerosis
Dr. Benedikt Weber, Swiss Centre for Regenerative Medicine, University Hospital Zurich. The most common cause of death among people in the western hemisphere is cardiovascular disease. In the majority of cases, the causes of the disease can be traced back to lesions in the arterial cell walls, so-called atherosclerotic plaque. At present, various animal models are used to investigate the origin of cardiovascular disease as well as for developing and testing new medication aimed at preventing/arresting or even curing the disease.
In this project, the research team have succeeded in developing a three-dimensional cell culture system from human atherosclerotic plaque (from material obtained following heart and blood vessel surgery) whereby the variety of cells involved in the disease are included in the model. The system is also capable of imitating the pulsatile blood vessel phenomena for simulating the biomechanical forces that play an important role in the origin of cardiovascular disease. This system provides a new biological "tool" for this vast research field that will help to avoid the necessity of using a large number of laboratory animals.
Project 135-13 |
|
|
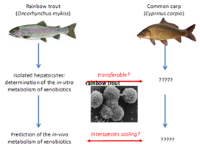 | In vitro alternatives to in vivo bioconcentration testing with fish: restricted to rainbow trout or broadly applicable?
Prof. Helmut Segner, Centre for Fish and Wildlife Medicine, University of Berne Programmes have been set up all around the world aimed at regulating the use of chemicals, such as pesticides, and for estimating the risk of their persistence, toxic effects and accumulaton, in particular bioaccumulation, in living creatures. One of the most commonly used models for such estimations is the bioaccumulation in fish, namely in their liver cells.
The project leader has already developed a liver cell culture system involving cells from trout, a cold-water fish, that can be used for in vitro testing (Project 108-07).
In this project the research team succeeded in demonstrating that it was possible to reproduce the data obtained from rainbow trout material (liver cells) using liver-cell cultures from carp (which are often used in Asian countries). This method offers the potential for totally eliminating or at least reducing the need for animal experimentation in this field.
Project 141-14 |
|
|
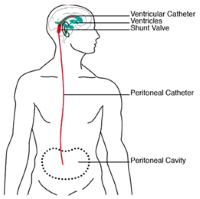 | Hydrocephalus simulator for testing of active ventriculoperitoneal shunts
Dr. Marianne Schmid Daners, Institute for Dynamic Systems and Control, ETH Zurich The valves and shunts that are used today in treating hydrocephalus patients are outdated. It often happens that the implanted system for draining excess brain fluid into the peritoneal cavity becomes blocked. As a rule, the efficiency of newly developed products is tested in large laboratory animals.
In order to render such testing superfluous a new platform has been developed that consists of a simulator that allows ventriculoperitoneal shunts to be tested in vitro.
Project 140-14 |
|
|
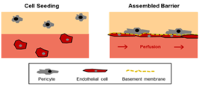 | An in vitro micro-vascular model mimicking the endothelial barrier
Dr. Marietta Herrmann, AO Research Institute, Davos The blood vessel walls, which are lined with endothelial cells that in turn are covered with pericytes, control the exchange of substances (nutrients, gases such as oxygen, etc.) in both directions (from the blood to tissue cells and vice versa). Cells too are transported through the walls of blood vessels, e.g. antibodies that spread from the blood to the tissues to eliminate invasive pathogens. As a rule, studies of the mechanisms involved in this transport of substances and cells rely on the use of laboratory animals, mainly transgenic mice.
The aim of this project was to establish a comprehensive in vitro vascular model including pericytes (microfluid chamber, basal membrane system, pericytes, etc.). Unfortunately, the technical difficulties were so overwhelming that the ambitious aims set by the research team could not be achieved.
Project 139-14 |
|
|
 | The Administrative Board regrets, that it is not possible to award new grants in 2017. The two sponsors of the 3R Research Foundation, namely the Federal Food Safety and Veterinary Office and Interpharma, will discontinue the support of the Foundation with a view to establishing a new 3R Competence Center.
|
|
|
 | On 25 April 2016 the Administrative Board approved the Foundation’s Annual Report for 2015, which describes its activities during last year, as well as the financial statements for 2015. A total amount of Fr. 321,990.05 was paid out for research projects. The final reports on four projects were received and for the last time four new projects were approved. The Foundation will not be able to allot any new research grants because it is unlikely to receive funds to continue the activity of the Foundation alongside the planned 3R Competence Centre.
Annual Report for 2015 | PDF version |
|
|
 | Validation of a new human in vitro model of microglia
Prof. Luis Filgueira, Department of Medicine, University of Fribourg Microglia refers to all the antibody cells that are stored between the nerve cells in the brain. A large number of laboratory animals, in particular mice, are used in experiments involving microglia from which such cells are isolated from the brain. Some years ago the authors of this project were able to demonstrate that microglia precursor cells could also be isolated from human blood and that, in vitro, they can subsequently be developed into mature microglia cells that are competent actors in the immune system.
The aim of this project was to validate this method and to publish a further paper to make the method better known. Convincing evidence was obtained that such human microglia cells produced in vitro are as functionally viable as microglia cells obtained from mice and from human brain tissue isolated from dead bodies.
Project 137-13 |
|
|
 | Development of an in vitro system to grow and investigate vascular endothelial cells under physiological flow
Prof. Robert Rieben, Department of Clinical Research, University of Berne As a rule, studies of pathogenic processes involving the blood vessel walls are carried out on laboratory animals. This is principally because it is not possible to simulate natural perfusion with blood in vitro since complete blood coagulates on the inner surfaces (endothelium) of the blood vessels.
The aim of this project was to establish blood vessel endothelium culture conditions that would enable researchers to simulate as closely as possible the most important flow parameters of blood (physiological flow and pressure conditions, shearing power, etc.) so that the inner surfaces of the blood vessels retain their physiological characteristics with the result that complete blood would not coagulate when coming into contact with them. The research team succeeded in establishing a micro-fluid system by which they could achieve the aims of the project. It should be possible for other research groups to adopt this relatively simple experimental concept without any problem.
Project 133-12 |
|
|
 | 3R-Info-Bulletin 56 New EEG analysis models are being developed in order to precisely and topographically locate pathological processes as well as to improve our understanding of the importance and the function of electrical brain activities. Experiments are being carried out to measure the electrical activity in the brain both through the skin and through the cranium with a high resolution using laboratory animals. Such experiments fall into category 3 with regard to suffering among the animals used. Dr. Gonzales Andino is proposing a new EEG analysis model that would enable such measurement to be made on the surface of the head. The principle of the new EEG model has been successfully tested and the results have been published.
3R-Info Bulletin 56 | Project 119-10 |
|
|
 | On 18. December 2015 the Administrative Board decided to cancel the call for grant applications in 2016. He regrets, that it is not possible to award new grants in 2016. The two sponsors of the 3R Research Foundation, namely the Federal Food Safety and Veterinary Office and Interpharma, will discontinue the support of the Foundation with a view to establishing a new 3R Competence Center.
|
|
|
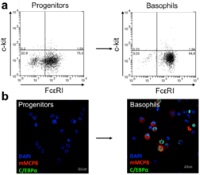 | 3R-Info-Bulletin 55 Although basophils constitute only around 0.5% of the leukocyte population in the blood, they appear to play an important role in the body's allergic reactions as well as in modulating and regulating the immune response. Research relating to the function of these cells usually involves mouse basophils. Owing to the fact that their concentration in the blood is extremely small, large numbers of mice are required for isolating a minimal quantity of such cells from the blood. Dr. Kaufmann's research team succeeded in producing immortalised basophil progenitor cells with which a virtually unlimited quantity of mature basophils can be obtained in vitro.
3R-Info Bulletin 55 | Project 127-11 |
|
|
 | Validation of a novel cell-based approach to study thyroidal physiology: Reduction and/or replacement of experiments with rodents
Dr. Gerasimos Sykiotis, Endocrinology, Diabetology and Metabolism Service, Vaud University Hospital, Lausanne, Switzerland Rats are normally used to study the function of the thyroid and the role of its secreted hormones. Owing to the small size of the organ, such experiments involve the consumption of many animals. Using stem cells, it has recently become possible to generate three-dimensionally in vitro a functionally-competent model of the hormone-producing part of the thyroid, namely, the follicle. The project aims to validate this new in-vitro model using murine cells, and to ascertain whether various pharmacological and genetic manipulations influence its formation and signal transmission during hormonal steerage in a reproducible manner.
Project 146-15 |
|
|
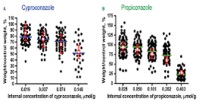 | Combining computational modelling with in-vitro cellular responses in order to predict chemical impact on fish growth
Prof. Kristin Schirmer, EAWAG, Dübendorf, Switzerland To examine the putatively negative side-effects of chemicals on the biological growth of fish and to determine the potentially poisonous impact of new substances that are intended for industrial and domestic use, hundreds of thousands of animals per year are legally subjected to experimental testing. The aim of this project is to develop a new computational model that is based upon toxicity data appertaining to cultured piscine cells. The comprehensive data-base that will be thereby derived will permit researchers in the future to conduct such testing computationally.
Project 145-15 |
|
|
 | Development of in-vitro three-dimensional multi-cellular culture models to study the role of heterotypic cellular interactions in colorectal cancer invasion
Prof. Curzio Rüegg, Department of Medicine, Chair of Pathology, University of Fribourg, Switzerland The survival chances of patients with cancer of the colon or the rectum are poor, owing primarily to the capacity of the cancerous cells to invade the surrounding tissue and to metastasize. Relatively little research has been conducted to elucidate the mechanisms that underlie the invasive process. The murine models that are currently available for studying the attributes of colorectal cancerous cells are of only limited use. Prof. Rüegg's research team has developed a promising new in-vitro model which can be used to investigate the interactions of the colorectal cancerous with stromal cells during the process of invasive growth. This model will now be rendered more sophisticated by including other cells that are implicated in the process (vascular cells, immune cells, etc.), as well as organ-like modules that better simulate cancer-cell biology.
Project 144-15 |
|
|
 | An advanced in-vitro model of pulmonary inflammation based on a novel lung-on-chip technology
Prof. Olivier Guenat, ARTORG Centre, Lung Regeneration Tech, University of Berne, Switzerland Inflammatory changes in lung tissue often lead to serious breathing problems. The complex processes that occur at the air-blood barrier, and which impair the exchange of gases and the flow of blood and influence the breathing mechanism, are normally simulated in animal experiments. The aim of this project is to create a lung model on a chip that will enable researchers to investigate various basic functions of the lung under the influence of pathological agencies, such as a post-traumatic inflammation.
Project 143-15 |
|
|
 | On 26 May 2015 the Administrative Board approved the 2014 Annual Report on the Foundation's activities as well as the financial statements for 2014. A total of Fr. 401,912.85 was paid out for research projects. Four new projects were approved and eight final project reports were submitted. The Administrative Board and the Evaluation Committee were re-elected for a further 4 year period.
Annual Report for 2014 | PDF version |
|
|
 | Non-invasive electrical monitoring of the population spiking activity in the central nervous system
Dr. Sara Gonzalez Andino, Micro-circuit Neuroscience Laboratory, EPFL, Federal Institute of Technology, Lausanne, Switzerland Electro-encephalography (EEG) measures electrical activity on the surface of the head. New EEG analysis models are being developed in order to precisely and topographically locate pathological processes as well as to improve our understanding of the importance and function of electrical brain activity. These new models are aimed at improving topographical resolution so that the electrical activity at even the topographical level of small nerve cell groups in the brain can be described. Experiments are being carried out to measure the electrical activity in the brain both through the skin and through the cranium with a high resolution using laboratory animals. Such experiments fall into category 3 with regard to suffering among the animals used. Dr. Gonzales Andino is proposing a new EEG analysis model that would enable such measurement to be made on the surface of the head. The principle of the new EEG model has been successfully tested and the results have been published. Further work must now be carried out to refine this method in order to achieve the aims defined at the outset.
Project 119-10 |
|
|
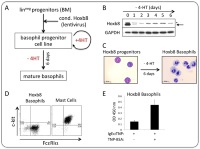 | Establishing a novel system for quantitative production of murine basophils in vitro
Prof. Thomas Kaufmann, Institute of Pharmacology, University of Berne, Switzerland White blood corpuscles are a mixture of various types of cells that serve to protect the system against pathogens that enter the body. It would appear that a subpopulation of these cells – basophils – plays an important role in allergic reactions as well as in the modulation and regulation of immune responses. Research into the functioning of these cells normally involves murine basophils but, owing to their extremely low concentration in the blood (0.5% of white corpuscles), a large number of mice are required in order to be able to isolate minimum quantities of the cells. Prof. Kaufmann's research team has succeeded in producing immortalised basophil precursor cells in vitro which can provide functioning basophils in almost unlimited quantities.
Project 127-11 |
|
|
 | Establishment of an in-vitro organ-slice defect model for meniscal repair in orthopaedic research
Prof. Ernst B. Hunziker, Centre of Regenerative Medicine for Skeletal Tissues, University of Berne Switzerland Injuries to the meniscus, especially in the human knee, are very frequent. Surgical removal of the damaged meniscus normally leads to arthritis in the joint after a few years. For this reason considerable efforts are being made to treat meniscus injuries biologically in order to avoid the later negative side-effects of surgical removal. Through tissue engineering using load-bearing material, regeneration cells and signal substances that regulate the healing processes, such ideas are normally tested on laboratory animals. The aim of this project was to establish a simple, cheap and standardised in-vitro meniscus model that would enable researchers to simulate the injury and the healing process in vitro on the basis of material obtained from slaughter houses (from bovine knee joints).
Project 130-11 |
|
|
 | Development of a cardiovascular simulator with auto-regulation
Prof. Stijn Vandenberghe, ARTORG, Biomedical Research Centre, University of Berne, Switzerland New materials and components such as heart valves, blood vessel walls, etc. are constantly being developed for heart surgery. The functioning, durability, tolerance, etc. of these components are tested on laboratory animals, which involves considerable suffering on their part. The aim of this project was to create an experimental cardiovascular machine which could be used for in vitro research and testing, including long-term testing. Prof. Vandenberghe's team has succeeded in building a realistic cardiovascular machine which accurately simulates the haemodynamic conditions and which can be regulated (using appropriate software) to simulate the various pathological circumstances.
Project 134-12 |
|
|
 | 3R-Info-Bulletin 54 The practical selection of specific antibodies for therapeutic, diagnostic and other purposes from a mixture of antibodies which are normally produced in animal experiments using repeated injections of immunogens is based on the use of phages (viruses) that are propagated in bacteria. They then express the desired target molecules on their surface (phage-display strategy), where they are recognised by the specific antibodies and then bind, thus enabling the researchers to select them. In this project, Prof. Heini and his team succeeded in developing an elegant, simplified phage-display strategy which requires fewer experimental steps and which can also be used in non-specialised laboratories. With this simplification and the possibility of the method being used more widely, the number of laboratory animals required can be considerably reduced.
3R-Info Bulletin 54 | Project 131-12 |
|
|
 | Validation of a human stem-cell based pluripotency test using a bioreactor-based culturing system instead of a murine model to effect the development of embryoid bodies into teratomas
Prof. Christian de Geyter, University Hospital Basle, Department of Biomedicine, 4031 Basle, Switzerland After stem cells from donor tissue have been isolated (or cultured) it must be checked whether they have maintained their typical capacity to differentiate into various types of tissue (pluripotency). According to international guidelines, such tests for pluripotency of stem cells are normally carried out using mice with a non-functional immune system.
The authors propose to develop a new bioreactor-based system which will enable researchers to examine the differentiation of stem cells into various types of tissue in vitro.
Project 142-14 |
|
|
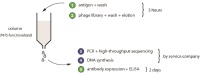 | Antibody phage selection strategy for application in non-specialized laboratories
Prof. Dr. Christian Heinis, Laboratory of Therapeutic Peptides and Proteins, EPFL, Lausanne, Switzerland Most antibodies used in research are still produced by immunizing animals. Prof. Heinis and his team succeeded in the development of an antibody scFv phage display library that may be distributed to laboratories free of charge and without any intellectual property (IP) constraints. From this library, antibodies to targets of choice can be isolated in vitro, omitting standard techniques based on animal immunization. In addition, they developed a phage display selection strategy with significantly fewer experimental steps that should facilitate the in vitro generation of affinity ligands by non-experts. The proposed method should replace animal experiments that are commonly performed to develop polyclonal and monoclonal antibodies.
Project 131-12 |
|
|
 | 3R-Info-Bulletin 53 The airway epithelium is the main port of entry for many respiratory pathogens and an important barrier to infection. Experimental systems that are suitable for studying basic virus-host interactions are scarce and are still preferentially performed in animal models. Prof. Thiel and his team succeeded to establish a unique in-vitro airway epithelial-cell culturing system that permits the molecular analysis of host-pathogen interactions at the port of entry of many respiratory pathogens.
3R-Info Bulletin 53 | Project 128-11 |
|
|
 | In vitro alternatives to in vivo bioconcentration testing with fish: restricted to rainbow trout or broadly applicable?
Prof. Helmut Segner, University of Berne, Centre for Fish and Wildlife Health, 3012 Berne, Switzerland Xenobiotic substances are chemical compounds that accumulate as foreign substances (e.g. toxins) in organisms where they are normally not found. According to OECD Guidelines (TG 305, Guidelines for Testing of Chemicals, Degradation and Accumulation), the potential risks of such substances must be tested on animals. A large number of animals are used for such tests.
The project leader has already developed a liver cell culture system using cells from trout, a cold-water fish, whereby the testing can be carried out in vitro (Project 108-07). The researchers now propose to adapt this system for liver cell cultures from carp, a warm-water fish, in order to enable the in vitro testing to be carried out in various other parts of the world.
Project 141-14 |
|
|
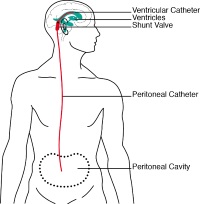 | Hydrocephalus simulator for testing active ventriculoperitoneal shunts
Dr. Marianne Schmid Daners, Zurich Federal Institute of Technology, Institute for Dynamic Systems and Control, 8092 Zurich, Switzerland The present generation of valves and shunts used for treating hydrocephalus is out-of-date. Blockages often occur in the artificial drainage system for transferring excess cerebral fluid from the brain to the abdominal cavity. As a rule, the reliability of new products or systems is tested on larger animals.
In order to alleviate the need for such animal testing, a new platform will be developed consisting of a simulator which can be used for testing ventriculoperitoneal shunts in vitro.
Project 140-14 |
|
|
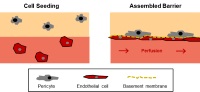 | An in vitro micro-vascular model mimicking the endothelial barrier
Dr. Marietta Herrmann, AO Research Institute Davos, 7270 Davos Platz, Switzerland The walls of the blood vessels that are lined with endothelial cells, which in turn are covered with pericytes, control the transfer of substances (nutrients, gases such as oxygen, etc.) in both directions (from the blood to the tissue cells and vice versa). Cells also pass through this barrier, e.g. antibody-producing cells, and access the tissue from the blood to eliminate pathogenic organisms that have entered the system. As a rule, studies of the mechanisms whereby substances and cells pass through the barrier involve the use of laboratory animals, mainly transgenic mice.
The aim of this research project is to develop a new in vitro culture system for the blood vessel wall whereby such studies can be carried out in vitro.
Project 139-14 |
|
|
 | On 6 May 2014 the Administrative Board approved the 2013 Annual Report on the Foundation's activities as well as the financial statements for 2013. A total of Fr. 568,479 was paid out for research projects. Four new projects were approved and three final project reports were submitted. Moreover, the Administrative Board decided on a major overhaul of the Administrative Board and the Evaluation Committee.
Annual Report for 2013 | PDF version |
|
|
 | 3R-Info-Bulletin 52 In humans, glioblastomas are the most common and most aggressive type of brain tumours. Animal experimentation as part of research into glioblastomas causes extreme suffering since it involves implanting a tumour in the brain of a mouse. Together with his research team at the University of Geneva, Dr. Olivier Preynat-Seauve has succeeded in developing a cell culture model whereby the interaction between the tumour cells and nerve tissue can be simulated. Through this model, experiments that cause considerable suffering to the mice should become superfluous.
3R-Info Bulletin 52 | Project 115-09 |
|
|
 | In vitro fish hepatocytes as source of metabolic clearance data in alternative approaches for the reduction or replacement of in vivo bioaccumulation testing with fish
Prof. Helmut Segner, Centre for Fish and Wildlife Health, Vetsuisse Faculty, University of Berne, Switzerland Numerous substances in daily use (cleaning fluids, cosmetics, medication, etc.) that are disposed of in waste water cannot be broken down naturally. Subsequently, they accumulate in various natural habitats a well as in animal species (bioaccumulation) with damaging effects on flora, fauna, the quality of our drinking water, etc. The problems caused by bioaccumulation are investigated using, among other things, trout. Prof. Segner and his team have succeeded in developing a new in-vitro testing method using liver cell cultures from these fish thus replacing testing in live animals.
Project 108-07 |
|
|
 | Organotypic slice cultures derived from brains obtained from slaughterhouses as an in vitro alternative for the investigation of neuroinfectious diseases in ruminants
Prof. Anna Oevermann, Vetsuisse Faculty, University of Berne, Switzerland Infections of the brain (or of the central nervous system), for example through bacteria (listeria), viruses (BSE) or active protein molecules (prions) normally lead to serious disease in humans or animals. So far, there is a lack of laboratory models, for example in vitro simulation, for researching such diseases. Prof. Oevermann has succeeded in developing a culture model for investigations in this area by using nerve tissue taken from animals obtained from slaughterhouses.
Project 116-09 |
|
|
 | Development of an in vitro model from embryonic stem cells for identifying tissue inflammation as a reaction to implanted material
Prof. Maria Wartenberg, Department of Molecular Cardiology, University Clinic, Friedrich Schiller University, Jena, Germany The development of new implant material for artificial hip joints, etc. involves, among other things, testing the rate of tolerance among recipients. Normally this is done using live animals. Prof. Wartenberg has succeeded in developing a tissue tolerance test using embryonic stem cells. This in vitro method provides valuable information as to potential tolerance in humans.
Project 117-09 |
|
|
 | Nerve-cell mimicking liposomes as an in vitro alternative for demonstrating the potency of toxins with multistep pathways such as Botulinum neurotoxins (BoNT)
Dr. Oliver G. Weingart, Institute of Food Sciences, Nutrition and Health, Zurich Federal Institute of Technology, Switzerland Botulinum neurotoxins are not only dangerous substances that are produced by bacteria in cases of infection and can lead to nerve paralysis, but are also used in a cosmetic preparation to eliminate wrinkles caused by ageing. Normally such neurotoxins are tested on laboratory animals. In this project, Dr. Weingart has achieved a major step towards developing a new in-vitro based efficacy test for these substances.
Project 125-11 |
|
|
 | Model development and validation to investigate myeloid cell homeostasis
Dr. Charaf Benarafa, Theodor Kocher Institute, University of Berne, Switzerland The blood's defence cells live for only a short time – a matter of hours – and for this reason many animals are required for research in this field. In order to replace these animals in the future, Dr. Benarafa and his team attempted to make such cells "immortal" so that many fewer or even no laboratory animals would need to be sacrificed. Unfortunately it was found that cells transformed in this way lose an important defence ability and certain other characteristics, with the result that they are no longer of use in research.
Project 126-11 |
|
|
 | Genetic modification of the human airway epithelium – a paradigmatic system to study host responses to human respiratory viruses
Prof. Volker Thiel, Institute of Immunbiology, St. Gallen Cantonal Hospital, Switzerland Many infectious diseases in humans start off in the airways, where germs manage to adhere to the airway epithelium and infect the victim's body (colds, influenza, etc.). Research in this field is based principally on animal experimentation. Prof. Thiel has succeeded in devising an in vitro epithelium cell model which will enable researchers to study the development of such human respiratory diseases in vitro.
Project 128-11 |
|
|
 | Using a microfluidic chamber to study mitochondrial transport in PTEN and SOCS3 dependent axonal regeneration
Prof. Zhigang He, Children’s Hospital Boston, USA Research into healing processes in damaged nerve fibers (axons) and testing of new substances for promoting such healing rely heavily on the use of laboratory animals. Prof. He and his team at the Boston Children's Hospital in the USA have succeeded in developing a new in-vitro system for investigating the early processes in the healing of such fibers using cell cultures.
Project 129-11 |
|
|
 | Identification of predictive in vitro markers for hematopoietic stem cell function
Prof. Matthias P. Lutolf, Laboratory of Stem Cell Bioengineering, Institute of Bioengineering, Lausanne Federal Institute of Technology, Switzerland Human haematopoietic stem cells have been successfully used in medicine to treat leukaemia. Before the treatment can be given, however, many tests must be carried out to determine the stem cell characteritics and various other functions and abilities of the cells, which normally involves laboratory animals. Prof. Lutolf has succeeded in developing a new in-vitro method for obtaining this information whereby some of the testing can be done in vitro using new markers.
Project 132-12 |
|
|
 | On 2 December 2013 the Administrative Board elected Joachim Eder, Member of the Council of States, as Chairman to the Administrative Board. He replaces Christine Egerszegi, Member of the Council of States, who resigned from the Administrative Board.
Administrative Board |
|
|
 | On 2 December 2013 the Administrative Board elected Dr. med. vet. Kaspar Jörger, Head of the Division Animal Protection of the (new) Federal Office for Food Safety and Veterinary Affairs to the Administrative Board. He replaces Prof. Hans Wyss, Director of the Federal Veterinary Office, who resigned from the Administrative Board.
Administrative Board |
|
|
 | 3R-Info-Bulletin 51 Foot-and-mouth disease is an affection of viral origin, which attacks cattle and swine. The consequences of infection are disastrous for individual animals. But also epidemiologically, an outbreak of the disease can spread like wildfire and is extremely difficult to eradicate. Hence, an effective vaccination of livestock is of paramount importance in controlling and combating outbreaks of the disease. The screening of potential vaccines is usually conducted in living animals and is a highly stressful experience for them. The aim of this project was to develop an in-vitro assay for the monitoring of vaccine potencies. Prof. Artur Summerfield’s research team at the Institute of Virology and Immunprophylaxis, Mittelhäusern, Switzerland have been successful in establishing a novel in-vitro system to measure the efficacies of vaccines against foot-and-mouth disease, thereby obviating the need for conventional serological testing in animals.
3R-Info Bulletin 51 | Project 113-08 |
|
|
 | Optimization of the nerve-cell-mimicking liposome assay as an in-vitro alternative for the detection of Clostridium-botulinum neurotoxins and for a validation of their presence in complex sample materials
Marc-André Avondet and Prof. Stephen Leib, Toxinology Group, Spiez Laboratory, FOCP, Switzerland Neurotoxins that are derived from the bacterium Clostridium botulinum are used medicinally to treat diverse disorders, such as dystonia, hyperhidrosis, strabism, chronic pain and headaches. More recently, a lucrative market for these toxins has been captured in the field of cosmetic surgery, in which they are applied as anti-wrinkle agents. For the quality control of new toxin batches, more than half a million mice per year are utilized in Europe and the USA alone. With the ultimate goal of entirely replacing such mouse-based assays, the investigators working on this project will further develop a new methodological approach that has been conceived using nerve-cell-mimicking liposomes.
Project 138-13 |
|
|
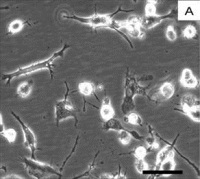 | Validation of a new human in-vitro model of microglia
Prof. Luis Filgueira, Department of Medicine, University of Fribourg, Switzerland The local resident defence cells of the brain are microglia. They are implicated in inflammatory responses of the brain and in protecting it against infection, as well as in the regenerative processes that follow injury or pathological degeneration (such as that associated with Alzheimer’s disease). With a view to elaborating novel therapeutic approaches to the treatment of brain diseases, the roles played by microglia in their development must be understood. To forward this goal, various animal models have been established. And, regrettably, even in-vitro studies cannot be conducted without the loss of animal life, since the necessary microglia can be obtained only from excised brains. The investigators who are working on this project have discovered that cells (monocytes) originating from human peripheral blood can be transformed into microglia. This finding opens up the possibility of establishing a human in-vitro model of microglia and thus of obviating the need for the sacrifice of animal life. In this project, the human in-vitro model of microglia will be validated.
Project 137-13 |
|
|
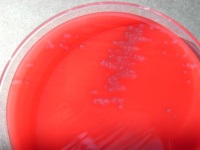 | Development of an in-vitro potency assay for the Clostridium-chauvoei vaccines: Replacement of the guinea-pig-challenge potency test
Prof. Joachim Frey, Institute of Veterinary Bacteriology, Vetsuisse Faculty, University of Berne, Switzerland Blackleg is a bacterial disease that affects cattle and sheep. It leads to extensive tissue necrosis, particularly in the muscles of the limbs. For the development of novel and/or improved vaccines to protect animals at risk, the efficacy of potential preparations are tested in vivo. The process of screening is usually conducted on guinea pigs and is a highly stressful one for the animals involved. The aim of this project is to develop a safe in-vitro screening system for routine laboratory use that would render the ubiquitous guinea-pig-challenge potency test obsolete.
Project 136-13 |
|
|
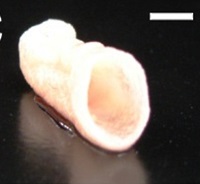 | In vitro engineering of a human cell-based three-dimensional dynamic model of atherosclerosis
Dr. Benedikt Weber, Swiss Center for Regenerative Medicine, University Hospital Zürich, Switzerland Cardiovascular diseases are a leading cause of death in the Western world. In most cases, the underlying lethal pathology is atherosclerosis, which targets the walls of the blood vessels. In addition to a genetic predisposition, the disease is often associated with obesity, excessive stress and nicotine addiction. To promote the development of novel therapeutic drugs for the treatment of atherosclerosis, it is necessary to understand the factors that contribute to its onset and progression, for which purpose various animal models are employed. In this project, the aim is to elaborate a clinically relevant in vitro model of atherosclerosis using human cells that are derived from surgical waste material.
Project 135-13 |
|
|
 | On 31 October 2013 the Administrative Board elected Dr. med. vet. Philippe Bugnon, who works for the Institute of Laboratory Animal Science, University of Zurich, to the Administrative Board. He replaces Silvia Matile-Steiner, who resigned from the Administrative Board.
Administrative Board |
|
|
 | On 7 May 2013 the Administrative Board approved the Foundation’s Annual Report for 2012, which describes its activities during last year, as well as the financial statements for 2012. A total amount of Fr. 619,000 was paid out for research projects. Four new projects were approved and the final reports on four projects were received.
Annual Report for 2012 | PDF version |
|
|
 | On 7 Mai 2013 the Administrative Board elected Dr. Birgit Ledermann, who works for Novartis Pharma Ltd., Basle (Deputy Corporate Animal Welfare Officer), to the Administrative Board. She replaces Dr. Markus Schmutz, who resigned from Novartis.
Administrative Board |
|
|
 | On 7 Mai 2013 the Administrative Board elected Claudia Mertens, dipl. phil. nat., Scientific Adviser of „Zürcher Tierschutz“, Zurich, to the Administrative Board. She replaces Dr. Franz P. Gruber, who resigned from the Administrative Board.
Administrative Board |
|
|
 | The Administrative Board has appointed Prof. Ernst B. Hunziker, head of the Center of Regenerative Medicine for Skeletal Tissues at the University of Berne, to take over as Scientific Adviser and Chairman of the Evaluation Committee on 1 March 2013.
Evaluation Committee |
|
|
 | Prof. Peter Maier retired at the end of 2012, having been involved with the 3R Research Foundation for 12 years. During that time he helped the Foundation develop into an institution that is recognised by academic researchers throughout Europe. The Administrative Board would like to extend their sincerest thanks to Prof. Maier for his fruitful work and wish him every success with his future career as a toxicologist at the University of Zurich.
|
|
|
 | 3R-Info-Bulletin 50 Prof. Peter Maier, Scientific Adviser of the 3R Research Foundation in Switzerland, reflects on the visibility, activity and future role of the Foundation.
Dr. Stefanie Schindler, an animal welfare expert, assesses the Foundation’s twenty-five years of funding.
To mark the Foundation’s 25th anniversary, the two authors describe the present situation with regard to animal experimentation in Switzerland (awareness of the Foundation’s work, limited funds) and the progress achieved so far concerning reduction und refinement.
In order to prevent an increase in the use of laboratory animals in the field of life sciences, the established research promotion involving live animals should specifically support aspects relevant to the 3R principles. A study is under way to analyse what the 3R Research Foundation has achieved over the past 25 years in various fields through its albeit limited funding; initial results are published.
3R-Info Bulletin 50 |
|
|
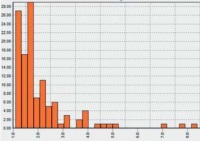 | Use of "moribund" stage in the acute fish toxicity test according to OECD guideline 203 and its effect on LC50 values
Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland The aim of this project is to define the final ”moribund” stage in the acute toxicity test in fish in order to introduce it as a criterion for interrupting the test under OECD guideline 203. Retrospective analysis of data and protocols from hundreds of toxicity tests carried out on fish has shown that by introducing the “moribund” criterion the suffering of the fish through the acute effects of a test substance could be reduced by up to 92 hours thanks to its earlier removal from the test. By introducing the “moribund” criterion, the LC50 value could be lowered, on average by a factor of 2, in up to half of the experiments. Attempts are now being made, in collaboration with the Swiss Federal Office for the Environment and various EU countries, to get the OECD protocol changed. This would help to achieve a significant reduction in the level of suffering among the fish used in this test.
Project 123-10 |
|
|
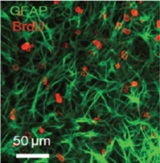 | A new in vitro model to study therapeutic approaches to improve spinal cord regeneration and repair after injury
Prof. Roman Chrast, Department of Medical Genetics, University of Lausanne, Lausanne, Switzerland This project aimed to examine the effect of various substances on spinal cord regeneration in vitro using mouse organotypic longitudinal, sagital slice cultures developed by the project leaders. The research team was also able to induce lesions similar to those seen in multiple sclerosis, thus allowing for functional and structural studies. Using this method it would be possible to avoid a large number of tests on live animals. Comparative in vivo and in vitro studies using potential new medicines remain to be carried out.
Project 121-10 |
|
|
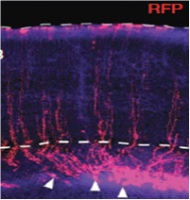 | Development of non-invasive strategies to study spinal cord disease, injury and repair
Prof. Denis Jabaudon, Neuroscience Department, University Medical Centre, University of Geneva, Geneva, Switzerland In this project, Prof. Jabaudon aimed to develop a method for the non-invasive injection of substances (e.g. plasmids) into the spinal cord of mice without using a surgical procedure. Such a method would result in a lower rate of mortality and less suffering among the laboratory animals used. The research team was able to demonstrate that the high-resolution ultrasound method for positioning the hypodermic needle for cortical injections was equally as accurate as the stereotactic method. On the other hand, the ultrasound method proved to be unsuitable for spinal injections. It was therefore not possible to establish the non-invasive injection method for use in the spinal cord.
Project 120-10 |
|
|
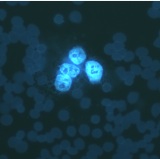 | Engineering of an in vitro hepatocyte tissue system for malaria liver infection research
Dr. Dalu Mancama, Biosciences Division, Systems Biology, CSIR, Pretoria, South Africa With this project, the researchers aimed to develop a hepatocyte tissue culture system using human liver cells for the purpose of studying the primary infection process of malaria (infection with plasmodium sporozoites isolated from the Anopheles mosquito) and the proliferation of the parasites in cultured liver cells. The ultimate aim would be to develop vaccines, prophylactic medicines and an acute chemotherapy without using laboratory animals. The main stages of the study were successfully completed. The in vitro culture (proliferation) of the asexual stage of Plasmodium falciparum with erythrocytes, the culture of human hepatocytes with sporozoites and the co-culture with infected erythrocytes were all successful. The rate of infection observed was low and was influenced by the culture conditions. Further verification of the results will be carried out.
Project 118-10 |
|
|
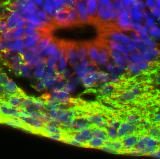 | Engineering of a human brain tumor model to replace animal experimentation
Prof. Olivier Preynat-Seauve, Department of Pathology and Immunology, University Medical Centre, University of Geneva, Geneva, Switzerland The aim of this project was to combine the delicate cell culture models developed by the project leader for brain tissue (engineered neural tissue, ENT, from embryonic stem cells) and glioblastomas (engineered glial tumors, EGT, from human tumor tissue), and thus to develop an in vitro system for studying glioblastomas and characterising their molecular structure. Such a system would replace in vivo methods that cause considerable suffering to the laboratory animals used, in particular in relation to the intracerebral or subcutaneous injection of human tumor cells in immuno-suppressed mice. The team were successful in establishing their in vitro system. Histo-pathological and molecular-biological studies were carried out on the reaction of the EGT transplanted into the ENT. It now remains to examine the various ways in which this model can be used.
Project 115-09 |
|
|
 | Generic in vitro evaluation assay for immunological correlates of protection, to replace animal challenge infection
Dr. Artur Summerfield and Dr. Kenneth McCullough, Institute of Virology and Immuno-prophylaxis (IVI), Mittelhäusern, Switzerland The aim of this project was to provide a reliable and fast in vitro test to replace in vivo testing by which live animals suffer considerably through being exposed to vaccines in order to match a potential vaccine to the current serotype of foot-and-mouth disease virus in the field. The researchers were able to measure a response specific to the virus type in cultures of specialised immune cells. By using genetically modified cells it was possible to further simplify and improve the test. This provides a scientific basis for developing reliable tests based on cell lines. The new test will now be further validated by a European consortium (FMD-DISCONVAC).
Project 113-08 |
|
|
 | In the interests of the welfare of laboratory animals For the past 25 years the foundation has been funding research into better or alternative methods to animal experimentation. To mark this anniversary it was organising a training course in the Technopark in Zurich, together with the Swiss Laboratory Animal Science Association. The media information published on 20.11.2012 provides the general public with an insight into the Foundation’s long commitment to animal protection and science.Media release (PDF)
Background information for the Jubilee (PDF) Six examples of research projects funded by the Foundation:
Observing rats using magnetic resonance imaging (MRI) (PDF) – Refine, Reduce (Project 82-02)
Behavioural studies to reveal mild pain in mice (PDF) – Refine (Project 71-00)
Cell culture systems for estimating the risk of inhaled (nano-) particles to replace inhalation experiments involving laboratory animals (PDF) – Reduce, Replace (Project 89-03)
It is possible to test the suitability of stem cells for treating brain damage using brain slices in cell culture (PDF) – Reduce, Replace (Project 103-06)
By using cells instead of pigs, it is possible to estimate the virulence of the classical swine fever virus (PDF) – Reduce (Project 105-06)
Testing for acute toxicity in fish: Fewer fish and less suffering (PDF) – Reduce, Refine (Project 114-08, Project 123-10)
|
|
|
| Scientific Manager The 3R Research Foundation is looking for a successor to Prof. Peter Maier who is resigning from his position on grounds of age at the end of the year.
Please submit your job application to the secre-tary’s office until 10 October 2012.
more... |
|
|
 | Development of a cardiovascular simulator with autoregulation
Prof. Dr. Stijn Vandenberghe, ARTORG Center for Biomedical Research, University of Berne, Switzerland Many studies are carried out in vivo for testing and gaining regulatory approval for cardiological devices. The aim of this project is to adapt a cardiovascular simulator to in vivo conditions in such a way that far fewer laboratory animals are required for testing such devices as blood-pumps, for example. The challenge in this case is to simulate self-regulating, clinically relevant mechanisms such as baroreflex (blood pressure and heart beat regulation) and the Frank-Starling mechanism (correlation between inflow and expulsion). The (preliminary) testing of devices using such further developed simulators should help to ensure that only the most promising devices are tested in vivo.
Project 134-12 |
|
|
 | Development of an vitro system to grow and investigate vascular endothelial cells under physiological flow
Prof. Dr. Robert Rieben, Department of Clinical Research, University of Berne, Switzerland The function of endothelial cells in the walls of blood vessels is often investigated in vivo, causing suffering to the laboratory animals involved. Conventional in vitro procedures simulate the physiological conditions with inadequate accuracy. This new in vitro experimental method is aimed at replacing many such in vivo studies. In the proposed in vitro model the pressure and shearing power of the pulsating blood, for example, are simulated. This system should make it possible to find answers to questions concerning ischaemia, reperfusion and transplantation that have so far been investigated in in vivo experiments involving considerable pain and suffering for the laboratory animals concerned.
Project 133-12 |
|
|
 | Identification of predictive in vitro markers for hematopoietic stem cell function
Prof. Dr. Matthias P. Lutolf, Institute of Bioengineering, EPFL, Swiss Federal Institute of Technology Lausanne, Switzerland In order to test whether hematopoietic stem cells (HSC) are capable of producing normal blood cells over a longer period of time, they are normally implanted into mice. Measurements are then made to see whether the HSC are able to reconstitute the blood system that has been destroyed through radiation. This procedure causes extreme suffering to the animals used and a large number of mice have to be sacrificed. In this project, Prof. Lutolf and his team intend to identify new cell markers to predict the long-term functioning of HSC that have been developed in vitro.
Project 132-12 |
|
|
 | Antibody phage selection strategy for application in non-specialized laboratories
Prof. Dr. Christian Heinis, Laboratory of Therapeutic Peptides and Proteins, EPFL, Swiss Federal Institute of Technology Lausanne, Switzerland Monoclonal antibodies for use in research continue to be produced using hundreds of thousands of rodents. The main reason for this is the complexity of the in vitro method and the limited use of commercially available phage libraries. In this project a new combination of techniques will be examined (high-throughput sequencing and DNA synthesis) with which the procedure can be carried out at a much lower cost. A sufficiently complex phage library should be made available to research laboratories without any restrictions and phage selection should be possible using fewer experimental steps. The simplified procedure could also be carried out in non-specialized laboratories. As a result, the simplification of antibody phage selection could help achieve a breakthrough in the application of this in vitro method, the principle of which has already been known to researchers for a number of years.
Project 131-12 |
|
|
 | Refinement goes together with Replacement On the occasion of its 25th Anniversary, the 3R Research Foundation is holding a joint meeting with the Swiss Laboratory Animal Science Association. This Continuing Education course will be held at the Technopark in Zurich on 19th and 20th November 2012. Over 40 speakers and lecturers from Europe including Switzerland, and from the U.S., have been invited. The papers to be presented at the sessions organised by the 3R Foundation will highlight progress in regard to the 3Rs. The European umbrella organisation of national consensus platforms for 3R alternatives (ecopa) and the Swiss Association of Toxicology (Swisstox) have been involved in putting together the programme for the meeting. Around 500 people are expected to attend.
Online registration
More information about this event | Program |
|
|
 | 3R-Info-Bulletin 49 Bacterial meningitis (BM) causes brain injury in the dentate gyrus (DG) of the hippocampus, with bacterial components and the host organ's inflammatory reaction contributing jointly to the insult. The researcher has succeeded in differentiating neural stem cells and precursor cells at different stages of development and simulating in them the reaction to the damaging effect of bacterial meningitis. It is likely that compromised brain areas can be repaired by grafting suitable stem/progenitor cells into brain tissue. This process has been successfully carried out in cultured brain slices in vitro. Thanks to the in vitro methods developed by the researcher, it will be possible in the future to carry out indicative preliminary examinations that are necessary to identify appropriate cells for transplantation, for example. It would only be necessary to use laboratory animals for the final confirmation of the in vitro findings.
3R-Info Bulletin 49 | Project 103-06 |
|
|
 | On 21 March 2012 the Administrative Board approved the Foun-dation’s Annual Report for 2011, which describes its activities during last year, as well as the financial statements for 2011. A total amount of Fr. 660,000 was paid out for research projects. Six new projects were approved and the final reports on eleven pro-jects were received.
Annual Report for 2011 | PDF version |
|
|
 | 3R-Info-Bulletin 48
includes a report on the completed project
Establishment of an organ ex-vivo tissue slice model for cardiovascular research, in particular for therapeutic atherosclerosis targeting
Prof. Patrick Hunziker, Clinic for Intensive Medicine, University of Basle, Switzerland In this project, aorta tissue of transgenic (ApoE-/-) mice was extracted and cultured ex vivo. A method was developed for obtaining the tissue and its subsequent on-line observation under a fluorescence microscope. It was possible to identify and characterise the sclerotic sections of the walls of the aorta (plaques) following perfusion of the aorta with specific markers. The time taken for changes to the cells could also be determined. The results obtained corresponded to a great extent to findings from studies using ApoE-/- mice, which shows that many studies, for example for preselecting potential new medicines, could be carried out ex vivo.
3R-Info Bulletin 48 | Project 111-08 |
|
|
 | On 17 January 2012 the Administrative Board elected Prof. Simon P. Hoerstrup, Head of the Swiss Centre for Regenerative Medicine (SCRM) at the University Hospital in Zurich and at the University of Zurich, to the Evaluation Committee.
Evaluation committee |
|
|
 | Establishment of an in-vitro organ-slice defect model for meniscal repair in orthopaedic research
Prof. Ernst B. Hunziker, Center of Regenerative Medicine for Skeletal Tissues, University of Berne, Switzerland Meniscus injuries are common and are caused by sport, among other things, or by osteoarthritis or rheumatoid arthritis in overweight patients. Sheep and goats are commonly used to test new treatments, such as replacing the damaged meniscus with synthetic material or even a prosthesis. In this project, Professor Hunziker and his team will be testing new methods in a specially developed chamber in which fine meniscus slices from cow joints obtained from slaughter-houses will be cultured. Investigation processes and incubation conditions will be improved (growth factors, microgranules, material used for the joint mucus membrane, etc.) until an optimum therapeutic concept, i.e. the repair of the meniscus lesion, is obtained. This method allows for the entire repair process to be followed for up to 6 weeks, thus obviating the need for up to 80% of laboratory animals in which a meniscus lesion has been artifically induced.
Project 130-11 |
|
|
 | Using a microfluidic chamber to study mitochondrial transport in PTEN and SOCS3 dependent axonal regeneration
Prof. Zhigang He, Children’s Hospital, Boston, USA The impaired regeneration of nerve cells in the central nervous system following damage to the spinal cord will be investigated in depth using, among other things, transgenic mouse models and will be verified in primates. Mechanistic investigations require the use of a large number of mice of many differently genetically modified strains and cause a great deal of suffering to the laboratory animals. Various therapies are being investigated at present. In this project a two-part microfluidic chamber will be used to study how axonal regeneration is inhibited. Cortical neurones that have been extracted from genetically modified mice will be seeded and, thanks to the special design of the chamber, axonal regeneration can be studied. This process will enable the researchers to identify new genes and structures that are involved in axonal regeneration. Potential pharmacological products will be tested. It should be possible to reduce the number of mice required for the in vivo testing, involving considerable suffering, by up to 50%.
Project 129-11 |
|
|
 | Genetic modification of the human airway epithelium – a paradigmatic system to study host responses to human respiratory viruses
Dr. Volker Thiel, Institute of Immunobiology, St. Gallen Cantonal Hospital, Switzerland The human airway epithelium is the point of access for many respiratory viruses, e.g. influenza viruses. In this project human airway epithelial cells acquired by bronchoscopy will be cultured instead of resorting to the frequently used rodent models (to determine mechanisms of infection). The biopsies contain various cell types that are typical of the epithelium, including cells and cilia that produce mucus. In order to determine the mechanisms (epithelium-virus interaction) the cultured cells will be genetically modified using established transduction methods (reporter proteins). As part of the project, the genetic modification methods will be further developed and standardised. This will allow the influence of certain proteins on virus infection rates to be examined in vitro. This procedure will not only lead to the replacement of test animals as well as those animals used in the rodent models but also enable the researchers to verify the validity of the results obtained using these animal models in relation to humans.
Project 128-11 |
|
|
 | Establishing a novel system for quantitative production of murine basophils in vitro
Prof. Thomas Kaufmann, Institute of Pharmacology, University of Berne, Switzerland Basophil blood cells fulfil non-redundant immunoregulatory and pro-inflammatory functions, despite constituting only 1% of all cells in the blood. It is not possible to culture such cells in vitro. For this reason, a large number of laboratory animals are required for the simplest functional or biochemical experiments using mouse models. Prof. Kaufmann intends to insert a genetic sequence (Hoxb8) into basophil precursor cells in mouse bone marrow in order to immortalise the cells and thus make it possible to culture them in vitro. Subsequently, the cells will be made to differentiate into mature basophils (using 4-hydroxytamoxifen). These cells can then be used for immunology testing (e.g. in transfection experiments). In this way it will be possible to replace a large number of mice, genetically modified mice and other animals required for their production.
Project 127-11 |
|
|
 | On 15 December 2011 the Administrative Board elected Nathalie Stieger, B. Econ. (St. Gallen), who works for F. Hoffmann-La Roche AG, Basle, to the Administrative Board. She replaces Silvia Matile-Steiner, who resigned from Hoffmann-La Roche.
Administrative Board |
|
|
 | An in vitro screening assay for stem/progenitor cells in organotypic hippocampal slice cultures: validation in an experimental model of infant rat pneumococcal meningitis
Prof. Stephen Leib, Institute for Infectious Diseases, University of Berne, Switzerland Cell changes were studied in organotypic hippocampal slice cultures in cell types that might play a role in brain damage and regeneration following bacterial meningitis. The research team succeeded in differentiating the stage of development of neuronal stem/progenitor cells cultured over a period of weeks. The suitability of such cells for transplantation and their behaviour in neuronal tissue were also studied. It was possible to show that the cells interact from a functional point of view with those in the hippocampus tissue slice. This in vitro method could be used for the relevant preliminary investigations and laboratory animals would only be required for the final confirmation of the in vitro findings.
Project 103-06 |
|
|
| The Administrative Board has decided that in 2012 applications will be dealt with in two stages in order to rationalise the procedure as well as to save applicants spending time on projects that are not likely to be approved for funding.
First of all a project outline should be submitted by 1 February 2012 at the latest.
If the Evaluation Committee considers the project to be relevant to the 3R principles it will then ask the applicant to submit a detailed application for funding.
Call for Grant Applications | Instructions concerning project outlines |
|
|
 | A new training device for taking blood and administering substances intravenously in rabbits in the form of an artificial rabbit’s ear made of silicon To minimise the animal’s discomfort, students need to practise taking blood from veins and arteries as well as administering substances in rabbits. They can use this newly developed silicon model of a rabbit’s ear for their initial attempts. The model ear is especially useful for compulsory basic courses for people who will be involved in laboratory experiments on live animals.
method M4 |
|
|
 | 3R-Info-Bulletin 47 It is important to be able to determine the possible concentration in fish of lipophilic contaminants that are released into the environment. In order to obtain such information without examining the fish themselves, it is necessary to determine to what extent such substances are metabolised, since this would influence their concentration in the living organism. It has been possible to demonstrate this metabolic processing in freshly isolated fish liver cells using reference substances. By standardising in vitro reports it has been possible to improve the validity of the results obtained using this animal-free method to such a degree that they are comparable with values obtained in fish.
3R-Info Bulletin 47 | Project 108-07 |
|
|
| On 28 September 2011 the supervisory authorities approved the Foundation’s revised regulations and the modification of the deed of foundation as decided by the Administrative Board on 30 March 2011.
Deed of foundation | Regulations |
|
|
 | On 30 March 2011 the Administrative Board approved the Annual Report for 2010 on the Foundation’s activities during that year, as well as the financial statements for the same year. A total of Fr. 728,600.00 was paid out for research projects. Seven new projects were approved for funding and the final reports for three completed projects were approved.
Annual Report for 2010 | PDF version |
|
|
 | 3R-Info-Bulletin 46
includes a report on the completed project
Evaluation of an in vitro model to identify host parameters associated with virulence of Toxoplasma gondii strains
Dr. Sushila D’Souza1, Pasteur Institute, Brussels, Belgium; 1present address: GSK Biologicals, Wavre, Belgium Toxoplasmosis, a disease which can be acute in man, is caused by Toxoplasma gondii (natural host: cats). The virulence of samples contaminated with Toxoplasma gondii is normally tested on mice. To replace these tests, which cause extreme suffering in the laboratory animals, Dr. D’Souza’s research team have cultured parasites from strains with various known levels of virulence together with human intestinal cells. A correlation between the level of virulence and the number and extent of the foci formation on the cell monolayer was observed, while a reverse correlation was seen with the degree of inhibition of the β-defensin2 proteins, which are responsible for defence against toxoplasmosis, in the intestinal cells. These changes could be used as an indicator for determining the virulence of Toxoplasma gondii without using laboratory animals.
3R-Info Bulletin 46 | Project 107-07 |
|
|
 | On 30 March 2011 the Administrative Board elected the Foundation’s officers for the coming 4 years. A new person on the Administrative Board is Dr. Markus Schmutz from Novartis Pharma AG in Basle. He replaces Prof. Paul Herrling, who decided to resign owing to his age.
Administrative Board |
|
|
 | Model development and validation to investigate myeloid cell homeostasis
Dr. Charaf Benarafa, Theodor Kocher Institute, University of Berne, Berne, Switzerland Neutrophil granulocytes play an important role in inflammatory diseases and in the body’s defence against pathogens. They are found only in minute quantities in the blood. In order to investigate their function a large number of laboratory mice, including transgenic types, have normally been used until now. Through the genetic manipulation (Hoxb8) of neutrophil precursor cells the research team intend to obtain functioning neutrophil granulocytes from the precursor cells. These cells will need to be characterised in order to determine that they are indeed behaving in the same way as differentiated cells. If the project is successful it will no longer be necessary to use a large number of laboratory mice nor to breed various types for laboratory experiments.
Project 126-11 |
|
|
 | Nerve-cell mimicking liposomes as an in vitro alternative for demonstrating the potency of toxins with multistep pathways such as Botulinum neurotoxins (BoNT)
Oliver G. Weingart, biologist, Institute of Food Sciences, Nutrition and Health, Zurich Federal Institute of Technology, Switzerland The registration authorities demand that the level potency be determined for each lot of toxins manufactured from living organisms before they are accepted for medical use. Until now, this has been done using the extremely painful LD50 test on mice. An alternative method involves using in part cell systems, but they are in many cases not sufficiently sensitive and difficult to reproduce. Using liposomes into which the individual steps in the potency pathway have been introduced should make up for this deficiency in the cell systems. It would then be possible to determine the potency level without using the LD50 test in mice.
Project 125-11 |
|
|
 | Magnetic Resonance Imaging (MRI) for the non-invasive assessment of lung inflammation and pulmonary function in the rat
Dr. Nicolau Beckmann, Novartis Pharma AG, Novartis Institute of Biomedical Research, Basle, Switzerland To develop treatments for asthma, induced inflammatory and fibrotic changes (early stages of asthma) have been characterised in rats using magnetic resonance imaging (MRI). Compared with conventional pulmonary function measurement and terminal histopathological analyses, the use of this non-invasive method means that the number of laboratory animals required can be reduced by up to 90%, and the condition of the individual animals can be monitored closely. From the point of view of animal welfare, this is a decisive advantage since, if the medication being tested has no effect, individual animals can be withdrawn from the experiment in the early stages of asthma.
Project 82-02 | extended abstract |
|
|
 | Non-mammalian Experimental Models for the study of bacterial infections (NEMO network)
Prof. Pierre Cosson,UMC, Faculty of Medicine, Department of Cellular Physiology and Metabolism, Geneva, Switzerland The mechanisms involved in bacterial infections and the cellular defence strategies can also be studied using amoeba and the fruit fly (Drosophila). With this specialised knowledge it would be possible to carry out certain infection experiments using amoeba and fruit flies instead of laboratory animals. In order to promote the use of this method, funding was provided for a 3-year platform for 5 research groups on the 3R Foundation’s website. It is to be hoped that other researchers will use the available models with amoeba and fruit flies for screening potentially effective antibiotics and thus reduce the number of conventional infection studies carried out using rodents.
Project 99-05 |
|
|
 | Organotypic CNS slice cultures as an in-vitro model for immune mediated tissue damage and repair in multiple sclerosis
Prof. Norbert Goebels1, Neuroimmunology Unit, Neurological Clinic, University Hospital, Zurich, Switzerland; 1present address: Heinrich-Heine University Düsseldorf, Department of Neurobiology, Germany The processes that lead to lesions in multiple sclerosis are often studied using an animal model (the EAE mouse model). As a part replacement, Prof. Goebels’ research team examined the immunological mechanisms of brain damage in cultured brain tissue slices of transgenic mice. They were able to demonstrate that antibodies and a complementary system cause demyelisation of the axons without damaging them. Antigen-specific cytotoxic (CD8) T-cells did cause collateral damage to the axons, however, but only when the tissue and the cytotoxic T-cells had been activated beforehand. To date it has not been possible to demonstrate this type of damage using the EAE animal model. This could result in a fall in popularity of the animal model and consequently a reduction in the number of EAE experiments carried out.
Project 101-06 |
|
|
 | Preparation and evaluation of lipoprotein fractions for the replacement of fetal bovine serum in cell culture media
Prof. Paul Honegger, Department of Physiology, University of Lausanne, Switzerland In most cases cell cultures require media that include fetal bovine serum in order to achieve optimal growth and to maintain cell function. Since the composition of the serum is not defined and varies from charge to charge, and since the acquisition of the serum from unborn calves should be avoided from an animal protection point of view, researchers have long been trying to find a defined serum replacement. Prof. Honegger’s research team have succeeded in demonstrating that, contrary to expectations, a macromolecular protein is not responsible for the stabilisation of cell function in the lipoprotein fraction. The results of their studies and their implications are described in detail in the extended summary and are available in Bulletin no. 45.
Project 109-08 | 3R-Info Bulletin 45 |
|
|
 | Development of an in vitro assay for screening of antischistosomal drugs
Prof. Jennifer Keiser, Swiss Tropical and Public Health Institute, University of Basle, Switzerland Infection by the parasite Schistosoma, which causes bilharziasis, can be treated in humans by targeting juvenile or adult Schistosoma parasites (in blood or organs respectively). At present, the parasites are obtained using mice or hamsters and the efficacy of potential treatments is tested in mice that have been infected with the parasite. Unlike the adult stages, the juvenile form can be cultured in vitro. The studies carried out by Prof. Keiser have shown that substances that were not effective against the juvenile stages of the parasite also showed no effect on the adult stages. Through improved in vitro testing it will no longer be necessary to carry out subsequent tests on laboratory animals.
Project 110-08 |
|
|
 | Reducing the number of fish and their suffering during acute toxicity testing of potential environmental pollutants (OECD Guideline no. 203).
Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland Through this project it has been possible to provide practical suggestions as to how the fish test involved in ecotoxicological studies (OECD guideline no. 203) can be improved from a 3R point of view. Thanks to retrospective analyses of hundreds of data series from fish tests and a mathematical simulation it has been possible to demonstrate that the number of fish used per test group could be reduced by 14% without any loss of quality in the results. A further reduction in the number of fish required can be achieved if the fish embryo test is used to determine the initial dose. The results obtained by Dr. Rufli’s research team have been discussed and accepted by selected experts in Europe and the USA. Since fish are caused level 3 suffering if the required maximum dose is used (and is effective), an attempt is being made to instigate a request from an OECD country (e.g. Switzerland) for a change in the OECD guidelines worldwide. The results of this project are summarised in Bulletin no. 43
Project 114-08 | 3R-Info Bulletin 43 |
|
|
 | 3R-Info-Bulletin 45 Cell culture methods enable the number of animal experiments in biological and industrial research to be reduced and in some cases replaced totally. Fetal calf serum must be added to most media to achieve optimum growth and to maintain cell functions. The composition of this serum is not defined, however, and varies from charge to charge. For reasons of animal protection, it would be preferable if the serum did not have to be taken from unborn calves. A partial step in this direction could be the identification of macro-molecular factors in the lipid protein-free fraction in fetal calf serum, as described in the Bulletin.
3R-Info Bulletin 45 | Project 109-08 |
|
|
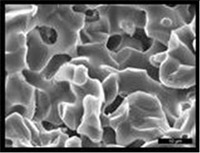 | Comparative in vitro and in vivo testing on biofilm formation on the surface of bone grafts
Dr. Martin Clauss, Orthopaedic Dept., Cantonal Hospital Liestal, Switzerland Infections associated with orthopaedic implants are difficult to treat because the resistant micro-organisms spread around the implant in a biofilm. For this reason it is important to develop materials that prevent the formation of this biofilm. These characteristics are being tested in guinea pigs. The research team has already developed an in vitro system and its practicability will now be examined in a comparative study of the guinea pig test and the in vitro test.
Project 124-10 |
|
|
 | Use of "moribund" stage in the acute fish toxicity test according to OECD guideline 203 and its effect on LC50 values
Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland In the acute fish toxicity test according to OECD guideline 203, LC50 is assessed in terms of the concentration of a test substance at which 50% of the fish die within an exposure period of 96 hours. In this project, sub-lethal effects on fish during the first 48 hours taken from reports on past testing are analysed and compared retrospectively to findings after 96 hours. The aim here is to be able to define the “moribund” stage whereby fish can be removed from the test at an earlier point without affecting the results. This would mean that the fish would suffer from the effects of the test substance concentrations for a shorter time.
Project 123-10 |
|
|
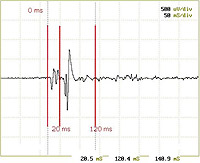 | Improved perioperative analgesia and reduced stress during recovery for the experimental animal: ultrasound-guided sciatic and femoral nerve block in sheep and quantitative assessment of block quality
Dr. Helene Rohrbach, Department of Clinical Veterinary Medicine, University of Berne, Switzerland Sheep are widely used for orthopaedic studies. In many cases the practicability (dose, duration of effect, method of administration) of the usual post-operative analgesic protocols is questionable, since sheep show hardly any outward signs of pain. In order to be sure of blocking pain more successfully, anaesthetics are administered regionally using a perineural catheter. The placing of the catheter near the sciatic and femoral nerves is monitored via ultra-sound, similar to the procedure used in humans. The efficacy of the analgesic is quantified. This should lead to the successful blockade of pain using lower doses and with fewer side-effects.
Project 122-10 |
|
|
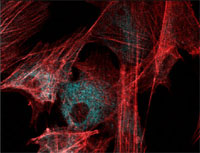 | 3R-Info-Bulletin 44 Classical swine fever is a highly contagious disease in pigs. The development of the disease depends on the virulence of the virus. The degree of virulence is important for monitoring and preventing the disease. Until now it could be assessed only through infected animals. Thanks to their detailed knowledge of the biochemical processes involved in the disease, Dr. Nicolas Ruggli and his research team have succeeded in developing tests involving cell cultures for replicating the virus as well as three tests to determine viral foci on a cell overlay, the macrophage infection rate and the production of IFN-alpha. A comparison with the virulence of 17 known viruses showed that, taken together, the three parameters provide a prediction of virulence that is equally as good as that obtained using live animals.
3R-Info Bulletin 44 | Project 105-06 |
|
|
 | On 1 June 2010 the Administrative Board approved the Annual Report on the activities of the Foundation during 2009 and the financial statements for the year. A total amount of Fr. 634,000.00 was paid out in research grants during 2009. Three new projects were approved and six final reports were acknowledged.
Annual Report for 2009 | PDF version |
|
|
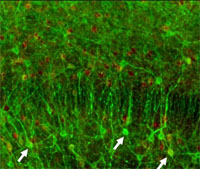 | A new in vitro model to study therapeutic approaches to improve spinal cord regeneration and repair after injury
Prof. Dr. Roman Chrast1 and Prof. Dr. Josef Kapfhammer2, 1Department of Medical Genetics, University of Lausanne, and 2Institute of Anatomy, University of Basle, Switzerland Spinal cord injuries (SCI) affect millions of people throughout the world every year, resulting in paraplegia and chronic pain. Until now animal models have been used to study therapeutic approaches to regeneration and repair. Such studies cause a high level of distress for the animals and the complexity of the in vivo processes reduces the reproducibility of the results. Using organotypic cultures of spinal cord slices that have been developed recently, it is possible to create lesions similar to SCI. The aim of this project is to characterise the function of the cells in the slice and to test the regenerative ability of two substances already used in clinical treatment, namely Nogo A and Cethrin. If the researchers succeed in achieving comparable results to those obtained in in vivo studies it will be possible to do away with a large number of experiments using laboratory animals.
Project 121-10 |
|
|
 | Development of non-invasive strategies to study spinal cord disease, injury and repair
Prof. Denis Jabaudon, Department of Basic Neurosciences, University of Geneva, Switzerland Worldwide, spinal cord injury and neurodegenerative diseases (e.g. amyotrophic lateral sclerosis) are the focus of intense and increasing research using laboratory animals with the aim of determining the processes involved and devising possible therapies. Such experiments using rats and mice often necessitate spinal cord intervention. These surgical procedures are time-consuming and cause a good deal of suffering for the animals; in addition, mortality is high (up to 30%). Many such surgical procedures could be replaced by the percutaneous introduction of a micropipette, using high-resolution ultrasound guidance, to inject dyes, genetic material or cells or for therapeutic intervention or placing electrodes. This procedure takes only 2-3 minutes and does not involve exposing the spinal cord. Consequently there is far less stress for the animal and the reduced mortality rate would mean that fewer animals would be needed.
Project 120-10 |
|
|
 | Non–invasive electrical monitoring of the population spiking activity in the central nervous system
Dr. Sara Gonzalez Andino, Department of Clinical Neuroscience, University of Geneva, Switzerland Various non-invasive methods (fMRI, optical imaging) are currently used for neuro/electrophysiological studies of the brain. None of these methods enables researchers to determine electrical activity in single cells or cell populations in the CNS, however. To date it has been necessary to implant electrodes in order to obtain more precise measurements. On the basis of the latest data from studies of epileptic patients and primates it is expected that a correlation will be found between scalp EEG and spiking activity (changes in the action potential) in cell populations. By comparing existing data series using appropriate software it has been possible to increase the spatial resolution of EEGs and to carry out many studies directly on humans. This should lead to a reduction in the number of primates required for invasive neurological investigations.
Project 119-10 |
|
|
 | Engineering of an in vitro hepatocyte tissue system for malaria liver infection research
Dr. Dalu Mancama, CSIR, Biosciences Division, Systems Biology, Pretoria, South Africa Malaria is a global infectious disease with a high mortality rate. Resistance to currently available drugs has led to a need to develop new products or vaccines. During the development of the disease, the interaction between parasites (Plasmodia) and host in the liver plays an important role. Plasmodium falciparum, which is responsible for malaria in humans, cannot be investigated using animal models. For this reason surrogate animal models are used because of the lack of human liver cell models in which the interaction between parasites and host can be studied. A novel tissue culture system (3D cultures) has been developed to increase the infectiousness of Plasmodium f. in human liver cells. . A novel tissue culture system with human liver cells (3D cultures) has been developed. The goal of the project is to enhance the infectiousness of Plasmodium f. in this system. If this is successful it will be possible to develop new treatments in liver cell cultures without the need to use laboratory animals
Project 118-10 |
|
|
 | 3R-Info-Bulletin 43 In testing fish for acute toxicity (OECD Protocol no. 203), the dosage of the substance tested is often too high or too low. Dr. Hans Rufli of ecotoxsolutions and 16 Swiss and foreign specialists analysed historical data on hundreds of chemicals used in agriculture and industry. Using retrospective computer simulations of testing procedures the team were able to demonstrate that the number of fish involved could be reduced by 15%; if the preliminary tests are carried out on fish embryos a further considerable reduction in the number of fish required can be achieved. At the same time, the validity of the results with regard to the toxicity of potential environmental pollutants remains high. The necessary steps have already been taken (via OECD) to propose this method on a global scale.
3R-Info Bulletin 43 | Project 114-08 |
|
|
 | Development of a novel multicellular 3-dimensional blood brain barrier in vitro model
Dr. Omolara Ogunshola, Institute of Veterinary Physiology, Vetsuisse Faculty, University of Zurich In this project a 3-dimensional model of the blood-brain barrier was simulated using endothelial cells, astrocytes and pericytes. In a collagen matrix the cells form a unit with a synergistic effect. The physiological reaction of this unit to various noxious substances was comparable to that seen in experiments involving live animals. This method therefore enables researchers to carry out mechanistic experiments on the function of the blood-brain barrier without using laboratory animals. The results obtained are summarised in Info Bulletin no. 42 and have already been published in part.
Project 93-04 | 3R-Info Bulletin 42 |
|
|
 | Development of a three-dimensional enteric cell culture model for in vitro studies of the intestinal eukaryotic parasites Cryptosporidium spp.
Prof. Alexander Mathis, Institute of Parasitology, University of Zurich Until now pathogenic intestinal parasites have been isolated in host animals (new-born calves) for subsequent experiments. In order to replace the need for live animals, a 3-dimensional model was used to culture epithelial enteric cells as a substrate for producing Cryptosporidium parvum parasites. The cell cultures were successfully established and can be considered as an in vitro model of the intestinal epithelium. The rate of production of the parasites in the cell cultures was extremely low, however, and limited to two days. The results have been set out in an extended summary.
Project 97-05 |
|
|
 | On 1 June 2010 the Administrative Board elected Dr. Ingrid Kohler from the Federal Veterinary Office in Berne to the Board and the Evaluation Committee. She will replace Ursula Moser, B.Sc., whose resignation has been received by the Board. The Board expressed their thanks to Miss Moser for her valuable contribution to the Foundation.
Administrative Board | Evaluation committee |
|
|
 | START-UP: Scientific and Technological issues in 3Rs Alternatives research in the process of drug development and union politics
Sponsored by: ecopa - European Consensus Platform for 3R Alternatives to Animal Experimentation This was a 2-year Support Action project (n° 201187) within FP7-HEALTH-2007-1.3-2 under the title “Bottlenecks in reduction, refinement and replacement of animal testing in pharmaceutical discovery and development”. The principal questions and approaches were identified at three preparatory meetings of experts. The meeting in Basle that focused on the 3R principles in relation to disease models in laboratory animals was organised in September 2008 by the 3R Research Foundation. Working meetings were held in Rome, Innsbruck and Budapest in the second year and possible improvements were presented for each of the 3R principles. A summary of all the proposals on how to implement the 3R principles was presented in May 2010 in a 230-page report that was submitted to the EU Committee. This report is intended as an aid to setting priorities in funding 3R-relevant methods in the FP8.
Poster on the project | Final Report |
|
|
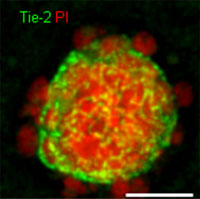 | 3R-Info-Bulletin 42 The task of the blood-brain barrier is to protect the brain but this function may be impaired by various medical conditions, e.g. stroke, Alzheimer’s, neuro-AIDS. Dr. Lara Ogunshola’s research team have succeeded in creating an in vitro 3-D model of the blood-brain barrier from three cell types – epithelial cells, astrocytes and pericytes. The physiological reaction to hypoxic conditions corresponded to the in vivo reaction. It was possible to examine complex processes such as synergistic interactions between various cell types. Thanks to the design of this research project it will be possible to find answers to many questions about the functioning of the blood-brain barrier without having to carry out experiments involving laboratory animals. (Project completed in March 2010)
3R-Info Bulletin 42 | Project 93-04 |
|
|
 | On 9 December 2009 the Administrative Board elected Dr. Martin Reist, Head of the Veterinary Public Health Institute (VPHI) at the Vetsuisse Faculty of the University of Berne, to the Evaluation Committee.
Evaluation committee |
|
|
 | Development of an in vitro model from embryonic stem cells for identifying tissue inflammation as a reaction to implanted material (INFPLANT)
Prof. Maria Wartenberg, Friedrich-Schiller University, Jena, and Prof. Heinrich Sauer, Justus-Liebig University, Giessen, Germany In the case of synthetic implants (teeth, blood vessels, heart valves) it is important that they are not rejected by the body’s immune system. Animal experiments are being carried out at present to clarify this question. In order to replace such experiments mouse embryonic stem cells will be used to develop an immune-competent cell culture system. Unlike with existing, simple in vitro procedures, it is expected that it will be possible to recognise inflammation and the formation of vessels.
Project 117-09 |
|
|
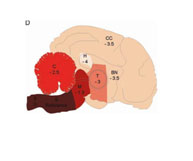 | Organotypic slice cultures derived from brains obtained from slaughterhouses as an in vitro alternative for the investigation of neuroinfectious diseases in ruminants
Dr. Anna Oevermann and Dr. Torsten Seuberlich, Vetsuisse Faculty, University of Berne, Switzerland Until now, it has only been possible to carry out investigations into the causes of spongiform encephalitis (e.g. prions) or listeriosis (listeria bacteria) in ruminants by using infected animals. In this project, tissue samples are taken from various areas of the brains of slaughtered, healthy ruminants (calves, sheep) and cultured in vitro. If structures and functions can be maintained in the tissue samples that are similar in vivo, it will be possible to gain a better insight into the causes (and mechanisms) of these diseases and to obtain infected material for examination without sacrificing live animals.
Project 116-09 |
|
|
 | Establishment of a murine syngeneic co-culture system of intestinal epithelial cells with intraepithelial T-lymphocyte subsets
Prof. Christoph Müller, Institute of Pathology, University of Berne, Switzerland In this project a co-culture system of human and mouse cells was developed. This enabled the research team to examine the interaction between intestinal epithelial cells and intra-epithelial lymphocytes (which differ from other T-lymphocytes). The results can be used directly for clinical application in humans. In this way, the number of animals required for mechanical experiments in the future will be considerably lower.
Project 98-05 |
|
|
 | 3R-Info-Bulletin 41 Using primary human cells, the company Epithelix has developed an airway epithelium (MucilAir) which can be cultured over several months. The tissue showed similar structural and functional characteristics to those of its in vivo counterpart. Dr. Song Huang and Mireille Caulfuty sought to determine the optimum culture conditions and established standard testing procedures (exposure time, duration of the experiment). The in vitro model serves to clarify the level of toxicity of matter and particles that can be taken in through the airway. (Project completed in May 2009).
3R-Info Bulletin 41 | Project 106-07 |
|
|
 | On 28 May 2009 the Administrative Board approved the Annual Report on the Foundation’s activities during 2008 as well as the financial statements for the year. A total of CHF 553,360.00 was allotted to research projects, while 6 new projects were approved for funding and two final reports were submitted.
Annual Report for 2008 | PDF version |
|
|
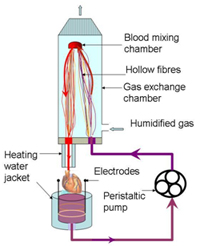 | 3R-Info-Bulletin 40 Dr. Anna Bogdanova and her team developed a mini-oxygenator for rodent hearts. This will enable investigations to be carried out on isolated hearts perfused with autologous blood (from the same animal) in connection with heart disease. Until now such investigations have involved considerable suffering for the animals used (heterotopic heart transplants).
3R-Info Bulletin 40 | Project 102-06 |
|
|
 | Engineering of a human brain tumor model to replace animal experimentation
Dr. Olivier Preynat, Department of Pathology and Immunology, Faculty of Medicine, University of Geneva The aim of this project is to combine brain-like tissue reconstructed from human stem cells (engineered neural tissue = ENT) with glioblastoma-like tumour tissue (engineered glial tumours = EGT). This should produce an in vitro model that can be used for examining the interactions between human brain and tumour cells in vitro and testing potential organ-specific cytostatic drugs. The researchers expect that the result will enable the number of corresponding animal models that involve a severe degree of suffering to be reduced.
Project 115-09 |
|
|
 | Adjuvanticity of microbial-derived particles and synthetic analogs in vitro
Prof. Elisabetta Padovan, Instituto Gulbenkian de Ciência, Oeiras, Portugal Certain adjuvants that stimulate the immune system may at the same time have toxic side-effects. In order to reduce the testing of such unwanted side-effects in animals, the research team have developed a three-tier cell culture system using human blood cells (monocytes, dendritic cells and T-cells), which can be used to identify potential unwanted toxic side-effects as well as the desired characteristics that stimulate the immune system. As a result, the use of animals in in vivo testing will become practically superfluous.
Project 92-04 |
|
|
 | Assessment of pain and stress in mice by monitoring gene expression changes
Dr. Paolo Cinelli, Institute of Laboratory Animal Science, University of Zurich This would lay the foundations for developing new ways of recognising pain. Two hundred genes were examined using micro-array technology and 27 genes were examined using the more sensitive RT-PCR (real-time polymerase chain reaction) method. No significant differences were observed between gene expression in selected areas of the brain in animals after and before surgical intervention.
Project 96-05 | 3R-Info Bulletin 39 |
|
|
 | Development of an in-vitro system for modeling bioaccumulation of neutral, ionizable, and metabolically Isolated, autologous blood-perfused heart: Replacement of heterotopic heart transplantation
Dr. Anna Bogdanova, Institute of Veterinary Physiology, University of Zurich In this project the research team successfully developed an ex vivo model of a rat heart that can be perfused using autologous blood (from the same animal). This method will enable tests to be carried out ex vivo which until now caused the laboratory animals considerable stress and pain (heterotopic heart transplants).
Project 102-06 | 3R-Info Bulletin 40 |
|
|
 | Development of in vitro strategies to propagate and characterize hemotrophic mycoplasmas
Prof. Regina Hofmann-Lehmann, Clinical Laboratory, Vetsuisse Faculty, University of Zurich The aim of this project was to replace the ethically dubious propagation of haemotrophic mycoplasmas in host animals (e.g. pigs) with an in vitro culture system for M. suis. Using a mycoplasma-specific medium, with the addition of fetal calf serum, porcine embryonic extract and transferrin, the team succeeded in maintaining continuous growth in M. suis. As a result, the characteristics of M.suis could be examined without prior propagation in host animals.
Project 104-06 |
|
|
 | Standardization and Pre-validation of MucilAir: A novel in vitro cell model of the human airway epithelium for testing acute and chronic effects of chemical compounds
Dr. Song Huang, Epithelix Sàrl, Plan-les-Ouates, Switzerland The MucilAir culture system, which consists of a human lung epithelium with ciliated epithelium, was successfully tested. Protocols were further standardised and a dose-effect relationship was obtained for each of 9 reference substances (from the EU AcuteTox project). The cultured cells showed a stable phenotype and retained their organ-specific characteristics.
Project 106-07 |
|
|
 | Establishment of an organ ex-vivo tissue slice model for cardiovascular research in particular for therapeutic atherosclerosis targeting
Prof. Patrick Hunziker and Dr. K. Bänziger, University Hospital, Basle Research into atherosclerosis (causes of the disease, possible therapies, nanomedicine) requires the use of a large number of laboratory animals. In Prof. Patrick Hunziker’s research team at the Basle University Hospital arteries are extracted from mice that lack the ApoE gene and human material, placed in a culture medium and then further examined. In this way it is possible not only to use fewer animals but also to determine whether differences exist between the clinical picture of the disease in mice and humans.
Project 111-08 |
|
|
| A novel in vitro model for holistic assessment and optimisation of engineered tissue for functional cartilage repair
Dr. Zhijie Luo and Prof. Jennifer Kirkham, Leeds Dental Institute, University of Leeds (UK) The aim of this project is, on the one hand, to examine synthetic cartilage from the point of view of function and characteristics and, on the other, to determine whether the new cartilage can fuse well with existing healthy cartilage. Normally, laboratory animals would be required for this purpose. The cell culture methods to be developed in this project should make it possible to carry out much of the research in vitro. It is also intended to further develop these alternative methods for use in routine testing.
Project 112-08 |
|
|
 | Generic in vitro evaluation assay for immunological correlates of protection, to replace animal challenge infection
Dr. Kenneth McCullough and Dr. A. Summerfield, Institute of Virology and Immunoprophylaxis (IVI), Mittelhäusern Foot-and-mouth disease antigens can vary enormously. In order to ensure that vaccination is successful it is necessary to tailor the vaccine to the latest viral sub-type and to test it on animals (challenge infection experiments). In order to replace the animal testing, a new and reliable in vitro test is being developed. The project is being carried out as part of a EU consortium with access to serum from vaccinated animals, reagents and mAbs. This means that international validation of the in vitro test will not require the use of additional animals.
Project 113-08 |
|
|
 | Reducing the number of fish and their suffering during acute toxicity testing of potential environmental pollutants (OECD Guideline no. 203).
Dr. Hans Rufli, ecotoxsolutions, Basle In acute toxicity testing on fish (OECD Protocol no. 203) excessively high or low doses of the test substance are often included in testing procedures. The aim of this project is to reduce the range of doses used through a retrospective analysis of historical data concerning hundreds of agricultural and industrial chemicals and by using statistical methods and simulated experiments, and at the same time to obtain equally reliable information about the toxicity of potential environmental pollutants. This would result in a drop of between 10 and 30% in the number of fish required for testing.
Project 114-08 | OECD Guideline 203 |
|
|
 | 3R-Info Bulletin 39 Dr. Paulo Cinelli and Igor Asner from the Institute of Laboratory Animal Science at the University of Zurich have tried to identify the genes involved in pain characterisation and its expression in mice. They expected to develop the basics for easy-to-use tests for pain recognition. This is a prerequisite for prescribing pain relief (if appropriate) at the right time and in an adequate dosage. Over 200 different genes in specific parts of the brain were examined using two different methods. So far no specific pain gene has been identified and no pain-dependent expression has been observed. The results provide a valuable basis for further research that has already been planned.
3R-Info Bulletin 39 | Project 96-05 |
|
|
 | On 11 December 2008 the Board elected Dr. Stefanie Schindler, a vet and biologist who is a member of the Animalfree Research Foundation in Zurich, to the Evaluation Committee.
Evaluation committee |
|
|
 | 3R-Info Bulletin 38 Prof. Marianne Geiser Kamber and her research team from the University of Berne isolated cells from the lungs of pigs for slaughter and developed organ-typical cultures, where in vivo conditions could be accurately replicated. Not only epithelial cells but also macrophages and epithelial secretion were obtained, the latter two being important for eliminating foreign particles from the lungs.
Not only will the use of this method lead to many animal tests becoming superfluous but it will also provide information about the processes involved in the harmful effects of substances.
3R-Info Bulletin 38 | Project 89-03 |
|
|
 | Scientific and technological issues in 3Rs alternatives research in the process of drug development and union politics The second Scientific Meeting under the auspices of the EU Start-Up project took place on 5 September 2008 at Novartis Ltd in Basle. Under the chairmanship of Prof. Peter Maier, 30 scientists from Europe and the USA, representing seven pharmaceutical companies, met to draw up proposals as to how the 3Rs could be further developed and put to use in disease models involving laboratory animals.
A summary of the successful meeting can be found in a report by Ecopa, which initiated the idea. The proposals should help to define future key points in EU projects relating to the 3Rs.
|
|
|
 | On 5 May 2008 the Administrative Board approved the Annual Report for 2007 concerning the Foundation’s activities during the previous year and the annual financial statements. Fr. 643 800.00 was paid out for research activities in 2007. In addition, the Foundation’s 20th anniversary was the focal point of the year’s activities.
Annual Report for 2007 | PDF version |
|
|
 | 3R-Info Bulletin 37 Dr. Beate Escher and her research team used the PAMPA (parallel artificial membrane permeability assay) in vitro system and developed the test further until the diffusion conditions largely corresponded to those found in the gills of fish. This new testing procedure avoids sacrificing a large number of fish and can now be used to determine the environmental dangers posed by chemicals (OECD Test 305).
3R-Info Bulletin 37 | Project 100-06 |
|
|
 | Evaluation of lipid fractions for the substitution of serum in cell culture media
Prof. Paul Honegger and Dr. Marie-Gabrielle Zurich, Department of Physiology, University of Lausanne, Switzerland Substituting fetal calf serum in tissue and cell culture media would lead to two major improvements: no fetuses would be required to obtain the serum and it would be easier to reproduce the results from the tissue and cell cultures. Lipid fractions would be a feasible substitute. Cell cultures are used to determine which fraction(s) are best suited to replace the serum.
Project 109-08 |
|
|
 | Development of an in-vitro assay for the screening of antischistosomal drugs
Prof. Jennifer Keiser, Swiss Tropical Institute, University of Basle, Switzerland Schistosomiasis is caused by various trematodes which are pathogenic in humans. Research to find potential drugs to treat this disease normally involves juvenile and adult schistosomes obtained from infected mice or hamsters. The aim of this project is to determine whether it is possible to replace the use of schistosomes by a test involving schistosomula isolated from the initial host (snails). If this proves possible, it would no longer be necessary to infect mammals to screen for possible drugs.
Project 110-08 |
|
|
 | On 5 May 2008 the Administrative Board elected Prof. Andrew Hemphill from the Institute of Parasitology of the University of Berne to the Evaluation Committee.
Evaluation committee |
|
|
 | The 3R Research Foundation is organising a meeting of selected experts from the pharmaceutical industry and universities, in Basle on 5 September 2008. This experts’ meeting is part of the ecopa Start-Up Project: Scientific and technological issues in 3Rs; Alternatives research in the process of drug development and union politics. Specific aspects of "3Rs bottlenecks in animal disease models" will be discussed.
Invitation - Registration Form |
|
|
 | In vitro fish hepatocytes as a source of metabolic clearance data in alternative approaches for the reduction or replacement of in vivo bioaccumulation testing with fish.
Prof. Helmut Segner, Fish and Wildlife Medicine, University of Berne Predictions concerning the bioaccumulation of pollutants in vitro are not reliable if the substances are metabolised in fish. This project is attempting to standardise cultures of isolated hepatocytes from rainbow trout in order to be able to accurately predict the metabolism of test substances in vitro; 5 different reference substances are being used.
Project 108-07 |
|
|
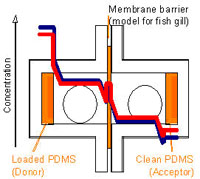 | Development of an in-vitro system for modeling bioaccumulation of neutral, ionizable, and metabolically active organic pollutants in fish
Dr. Beate Escher, Environmental Toxicology, EAWAG , Dübendorf It is essential to be able to determine the potential bioconcentration of pollutants in fish for assessing environmental risks (OECD Test 305). A large number of fish is required for this purpose.
The PAMPA (parallel artificial membrane permeability assay) in vitro system was further developed as part of this project. Using a selected artificial membrane (polydimethylsiloxane), an optimum stirring technique (oxygenation) and appropriate calculation methods, the research team succeeded in simulating the diffusion conditions that exist in the gills of fish. With the exception of substances that were metabolised in the fish, the level of permeability of 14 reference substances corresponded closely to the rate of elimination measured in vivo.
Project 100-06 |
|
|
 | In vitro replica of the inner surface of the lungs, for the study of particle-cell interaction.
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne This project studied ways of culturing tracheal epithelial cells and macrophages from porcine lungs. This facilitates the in vitro clarification of the adverse or therapeutic effects of nanoparticles in the lung. Consequently, inhalation studies in laboratory animals (mostly rodents) are unnecessary, or at least the number of such studies can be drastically reduced. In order to characterise the effects of aerosols, morphological and physiological changes in the cells were measured, including cilia activity, the production of cytokines and the extent of necrotic cell death. These studies will be continued as part of the international POLYOSA project.
Project 89-03 |
|
|
 | On 6 December 2007 the Administrative Board accepted the resignation of Dr. Hugo Wick as Chairman and thanked him warmly for his untiring work on its behalf.
It subsequently elected Mrs. Christine Egerszegi-Obrist, member of the Radical Democrat Party and of the Council of States and formerly Deputy Chairwoman, to succeed him.
Administrative Board |
|
|
 | Also on 6 December 2007, the Board accepted the resignation of Dr. Peter Heer and thanked him for his services.
Mrs. Silvia Matile-Steiner, a lawyer with F. Hoffmann-La Roche AG in Basle, was elected to the Administrative Board in his place.
Administrative Board |
|
|
| Development of a QSAR model for classification and prediction of baseline toxicity and of uncoupling of energy transduction The toxicity of chemical substances in the environment may depend on their effects on energy metabolism in cells (uncoupling of oxidative phosphorylisation). A quantitative structure-activity relationship (QSAR) can be calculated for such substances. This enables the toxicity of the substances to be quantified, thus eliminating the need for a large proportion of experiments involving laboratory animals.
Project 95-05 |
|
|
 | A non-mammalian system to study bacterial infections The research team has succeeded in determining the virulence of bacteria in single-cell amoebae (Dictyostelium). The virulence of selected bacteria in amoebae and rodents was shown to be similar. The same genes are involved in immunity mechanisms in amoebae and mammals. As a result, many of the most invasive infection experiments are no longer necessary.
A generally comprehensible summary of the results was included in 3R-Info Bulletin 36.
Project 90-03 |
|
|
 | 3R-Info Bulletin 36 Experiments using mice and rats to examine the virulence of bacteria are highly invasive for the animals. The summary in the Bulletin indicates that many such experiments can be carried out as effectively using amoebae (Dictyostelium) as well as flies (Drosophila melanogaster) or worms (Caenorhabitis elegans). The system in the amoeba is extremely easy to use and can be adapted to the special requirements of bacteria (e.g. including fish pathogens).
3R-Info Bulletin 36 |
|
|
 | 20th Anniversary of the 3R Research Foundation
“Good science with less animal experimentation“ On 29 August 2007 the 3R Research Foundation held a press conference in Berne where the new 3R brochure was presented.Hugo Wick, the President of the Foundation, Christine Egerszegi, President of the National Council and Vice-President of the Foundation, Thomas Hartung, Director of ECVAM, Hans Wyss, Director of the Federal Veterinary Office and Thomas Cueni, Secretary General of Interpharma, each illustrated from his or her own viewpoint the past 20 years of pioneering research and dialogue in connection with animal protection and science.
press documentation (PDF in German) | press release (PDF in German) |
|
|
 | To mark this 20th anniversary, the 3R Research Foundation and the Swiss Laboratory Animal Science Association (SGV) organised a scientific meeting, in collaboration with the Association for Training in Laboratory Animal Welfare and the Animal Keepers’and Technical Staff Interest Group, at the Zurich-Irchel University on 3 and 4 September 2007. The 2-day meeting was attended by over 400 people; 39 speakers presented papers and led workshops.The 3R sessions that were organised by the 3R Research Foundation were attended by over 260 people. The 14 invited speakers, who presented various interdisciplinary topics, captivated their audience.
Photos from the meeting |
|
|
 | All participants at the scientific meeting to mark the 20th Anniversary of the 3R Research Foundation and the 20th Anniversary of the Swiss Laboratory Animal Science Association (SGV) were given summaries of the papers presented. The first day of the meeting was devoted to the topic of “Humane Endpoints“. Opinions and experience were exchanged in two parallel sessions and 4 workshops. On the second day, the 3Rs were debated from every point of view.The summaries of the papers given have been collated in a 70-page brochure. The various papers given in the 3R sessions start on page 29.
summaries (PDF) |
|
|
 | Good science with less animal experimentation In the form of a generally understandable and up-to-date publication, the brochure presents the 3R principles (replace, reduce, refine), whose implementation has been promoted by the 3R Research Foundation during the past 20 years.
On 36 pages the brochure deals with the problems and limits of replacing animal experiments by alternative methods; it also describes past achievements as well as future possibilities and expectations.
The brochure can be obtained free of charge from the Foundation Secretariat in German, French and English. Enquiries to: secretary.3r@bluewin.ch.
Brochure (PDF) |
|
|
 | In order to celebrate the 20th anniversary of the 3R Research Foundation, a number of reports on successfully completed as well as ongoing projects are summarized in a special issue of ALTEX.
Editors: Peter Maier and Franz P. Gruber The 20 selected projects, which have been carried out in the course of the Foundation’s 20 years of activities, testify to the sustainability of support received. The abstracts of the ongoing 17 projects point to the results expected in the future.
The special issue can be obtained free of charge from the Foundation Secretariat. Enquiries to: secretary.3r@bluewin.ch.
Special issue of ALTEX (PDF) |
|
|
 | Evaluation of an in vitro model to identify host parameters associated with virulence of Toxoplasma gondii strains.
Dr. S. D’Souza and V. Marambudi, Pasteur Institute, Brussels, Belgium Toxoplasmosis is an infectious disease in humans. It is caused by the Toxoplasma gondii parasites. The virulence of Toxoplasma gondii strains is to be determined in cultures with human bowel cells. At present only a test with mice is available for the determination of the infection’s virulence. In future this could be replaced by a cell culture test.
Project 107-07 |
|
|
 | Standardization and Prevalidation of MucilAir: A novel in vitro model of the human airway epithelium for testing acute and chronic effects of chemical compounds.
Dr. S. Huang, Epithelix Sàrl, Geneva A worldwide recognized toxicity investigation of chemicals can only be carried out by means of validated tests. In order to investigate lung toxicity, a culture system using cells from the human airway epithelium has been developed by the Epithelix Company (MucilAir). In a first step, this in vitro procedure must pass a prevalidation test so that it can be adopted by the ECVAM in Europe-wide validation proceedings. Only as a validated procedure can it then be practically applied e.g. in the EU REACH Program.
Project 106-07 |
|
|
 | On 26 March 2007 the Administrative Board approved the Annual Report for 2006 concerning the Foundation’s activities during the previous year and the annual financial statements. Fr. 726 000.- was paid out for research activities
Annual Report for 2006 | PDF version |
|
|
 | 3R-Info Bulletin 35 Prof. Gert Fricker and his colleagues, Valeska Reichel and Carsten Baehr, have developed a cell culture system in which the barrier functions in an intact organism (Choroid plexus epithelium) are closely replicated. As a result, it possible to investigate the exchange of substances between cerebrospinal fluid and blood in vitro, thus obviating the need to use animals in experiments.
3R-Info Bulletin 35 | Project 91-04 |
|
|
 | A meeting will be held on 3-4 September 2007 at the Zurich-Irchel University in Zurich to mark the 3R Research Foundation’s 20th anniversary, at the same time as the 20th anniversary of the Swiss Laboratory Animal Science Association (SGV). This meeting will be organised in collaboration with the Association for Training in Laboratory Animal Welfare and the Animal Keepers’and Technical Staff Interest Group.
Registration for the meeting:
Register via the SGV website. Up-to-date information about the programme you will find right here .
If you want to participate only on Tuesday 4.9.2007, you are not a member of the SGV and do not require confirmation of having attended the meeting as part of your further training, the Foundation will pay your registration fee. Please register even so to obtain a name-badge for access.
|
|
|
 | The NEMO (non-mammalian experimental models for the study of bacterial infections) network is made up of working groups that carry out studies of bacterial infections in non-mammalian organisms (amoeba, Drosophila). In February 2005 five research laboratories at Swiss and French universities founded the NEMO network with funding from the 3R Research Foundation. Meetings were held in 2006 and 2007 and collaboration between the various working groups was started. The plan is now to expand this network to cover the whole of Europe.Anyone interested should contact:
Prof. Pierre Cosson,
Dept. of Cell Physiology and Metabolism
Centre Medical Universitaire
1 rue Michel Serve
CH-1211 Geneva 4
Switzerland
Aims and activities of the NEMO network |
|
|
 | Establishment of an in vitro system for the prediction of the degree of virulence of classical swine fever virus isolates
Dr. Nicolas Ruggli, Institute for Viral Infections and Immune Prophylaxy (IVI), Mittelhäusern In the case of an outbreak of CSF the whole herd is slaughtered as a preventive measure. For this reason a differentiated diagnosis of the virulence of the infection is necessary. In this project an attempt is made to determine the virulence of the swine fever in cell cultures, which alleviates the need to use live animals for that purpose.
Project 105-06 |
|
|
 | Development of in vitro strategies to propagate and characterize hemotrophic mycoplasmas
Prof. Regina Hofmann-Lehmann, University of Zurich The contagiousness of hemoplasmas for humans and animals needs further investigation. In order to avoid using animals to obtain these cell-wall-free bacteria, methods have been developed to produce these hemoplasmas in vitro.
Project 104-06 |
|
|
 | An in vitro Model of Central Nervous System Infection & Regeneration: Neuronal Stem Cells as Targets of Brain Damage & Regenerative Therapies in Bacterial Meningitis
Prof. Stephen Leib, University of Berne Bacterial meningitis in humans often causes brain damage and can be fatal. In order to develop therapies, the mechanisms of the infection need to be investigated further. At present this is done using live laboratory animals. With the help of cultured neuronal stem cells and organ sections obtained from rats, at least part of these investigations could be carried out in vitro.
Project 103-06 |
|
|
 | 3R-Info Bulletin 34 Dr. Yara Banz and Dr. Robert Rieben of the University of Berne have succeeded in maintaining the natural anticoagulation effect of endothelial cells in cell cultures. This will enable certain questions to be answered without resorting to the use of animals in the laboratory.
3R-Info Bulletin 34 | Project 81-02 |
|
|
 | On 19 December 2006 the Administrative Board accepted the resignation of Dr. Alfred Schweizer from the Evaluation Committee. The Board thanked him for his many years of valuable service as chairman.
Prof. Peter Maier, scientific advisor to the Foundation, has been elected as the new chairman of the Evaluation Committee.
Evaluation committee |
|
|
 | On 19 December 2006 the Administrative Board also accepted the resignation of Dr. Franz P. Gruber from the Evaluation Committee. He was also thanked for his hard work on behalf of the Foundation.
Susanne Scheiwiller, a biologist and joint director of FFVFF in Zurich, was elected to replace Dr. Gruber.
Evaluation committee |
|
|
 | 3R Project 79-01 In their recently published article, T. Kröber and P.M. Guerin of the Zoological Institute at the University of Neuchâtel describe how ticks can be fed in vitro.
T. Kröber and P.M. Guerin (2006) An in vitro feeding assay to test acaricides for control of hard ticks. Pest Management Science. 63, 17-22.
In the on-line press reviews at Télévision Suisse Romande and TIMESONLINE from 6th November 2006 and in Frankfurter Allgemeine Zeitung from 3rd January 2007 this research is claimed to represent a breakthrough in replacing the use of animals.
3R-Info Bulletin 27 | Project 79-01 |
|
|
 | 3R-Info Bulletin 33 Prof. W. Pichler, University Hospital, Berne, demonstrates that even substances that are not known to be hapten-carrier com-plexes can cause T-cells to divide, i.e. substances can trigger an immune response solely through their chemical structure.
3R-Info Bulletin 33 | Project 80-01 |
|
|
 | A module on recognising pain has been added to the 3R Training course A new module entitled “Postoperative pain recognition in animals“ has been added to the further training course offered on the internet by the 3R Research Foundation, which is recognised by the cantonal authorities. Module devised by: Prof. P. Flecknell, University of Newcastle.
more... | 3r-training.tierversuch.ch |
|
|
 | Are you looking for cell culture media for serum-free culture systems? Would you like to register a new, serum-free medium? An interactive database is now available on the internet to provide all such information to those interested.
more... | www.sefrec.com |
|
|
 | 3R-Info-Bulletin 32 Dr. N. Beckmann from Novartis Pharma in Basle demonstrates, using asthma research as an example, how the number of animals used for testing can be dramatically reduced, the distress caused can be attenuated and the length of the experiments can be shortened, by applying magnetic resonance imaging (MRI).
3R-Info Bulletin 32 | Project 82-02 |
|
|
 | On 21 March 2006 the Administrative Board approved the Annual Report for 2005 concerning the Foundation’s activities during the previous year and the annual financial statements. Fr. 540 000.- was paid out for research activities
Annual Report for 2005 |
|
|
 | Induction of a primary T-cell-mediated immune response against drugs and drug metabolites in vitro.
Prof. Werner Pichler, University Hospital, Berne Results have shown that the immunogenic (as well as allergenic) potential of a potential drug can be assessed using a T-cell activation test. The test involving human cells requires further development before it can be used routinely in pre-clinical safety testing.
Project 80-01 |
|
|
 | In vitro cultivation of Neospora caninum and Toxoplasma gondii oöcysts
Prof. Andrew Hemphill, University of Berne The ultimate aim of the project was to cultivate this stage of Neospora caninum and Toxoplasma gondii in vitro, thus eliminating the need to cultivate them in dogs. The project served to expand the necessary basic knowledge and opened the way to achieving the aim of the research.
Project 85-03 |
|
|
| On 21 March 2006 the Administrative Board accepted the resignation of Prof. Max Gassmann of the Institute of Physiology, University of Zurich-Irchel, from the Evaluation Committee. The Board expressed its sincerest thanks for Prof. Gassmann’s work on behalf of the Foundation.
Dr. Kurt Lingenhöhl, head of laboratory at Novartis Pharma AG (Novartis Institutes for Biomedical Research) was elected to the Evaluation Committee.
Evaluation Committee |
|
|
| Applications for sponsorship of projects concerning the development or improvement of testing methods in the field of regulatory toxicology are sponsored only if they present a new concept or aim to develop a new method which is subsequently approved in an international validation process (appendix).
more... |
|
|
 | Development of an in-vitro system for modelling bioaccumulation of neutral, ionizable, and metabolically active organic pollutants in fish
Dr. Beate Escher, EAWAG, Dübendorf With regard to chemicals that are new on the market, toxicity testing must include determining the bioconcentration in fish according to OECD guideline 305. This project aims to develop a method of determining the level of bioconcentration using a parallel artificial membrane permeability assay = PAMPA) instead of live fish. This simplified approach should mimic in vivo conditions well since the principal way fish ingest toxic substances is through (passive) diffusion through their gills.
Project 100-06 |
|
|
 | Organotypic CNS slice cultures as an in-vitro model for immune mediated tissue damage and repair in multiple sclerosis
Prof. Norbert Goebels, University Hospital, Zurich The processes that lead to the development of multiple sclerosis are still largely unknown. The experimental autoimmune encephalomyelitis (EAE) model is used in research. This animal model cannot portray all aspects of the disease, however. By using organotypic CNS slice cultures from mice, together with electrophysiological measurements it is possible to examine the remaining aspects in vitro and replace a large proportion of the EAE experiments.
Project 101-06 |
|
|
 | Isolated, autologous blood-perfused heart: Replacement of heterotopic heart transplantation
Dr. Anna Bogdanova, University of Zurich The pathological processes that occur with ischaemia (complete hypoxia) and subsequent blood perfusion (e.g. of the brain and the heart) are still not fully understood. In order for these experiments to be carried out without resorting to the use of animals (heterotopic heart transplants) a heart-lung machine is necessary for the much smaller hearts of rodents and for blood perfusion (extra-corporal). The aim of this project is to develop such a machine.
Project 102-06 |

![]()






































































































































































