 |
de | fr | en print view![]()
3R-INFO-BULLETIN 33
September 2006
Author

The project was carried out by Prof. Dr. Werner J. Pichler, Head of Allergology at the University Hospital in Bern and his collaborators (Andreas Beeler, Jan Depta, Olivier Engler, Daniel Yerli). His group has been investigating drug hypersensitivity for the last 15 years. They were able to decipher the pathomechanisms of various drug hypersensitivity reactions and defined an alternative pathway for drugs able to stimulate the immune system (p-i concept).
Current address:
Prof. Dr. Werner J. Pichler
werner.pichler@insel.ch
Dept. for Rheumatology and clin. Immunology/Allergology
Inselspital
University of Bern
CH-3010 Bern
Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Predicting drug hypersensitivity by in vitro tests
Before a compound such as a chemical or drug is put on the market, it would be ideal to be able to determine its allergic potential[*]. However, due to species differences and the individual response of each person, available in vitro and in vivo tests cannot detect all possible interactions between compounds and the human immune system. In project 80-01, the 3R Research Foundation supported the development of an in vitro test with human blood cells (T cells) which covers a pathway (p-i concept) which is still undetectable in animal tests. The results of the project demonstrate two aspects: a) a possible way of developing a predictive test and b) the obstacles to overcome to reach this goal - the highly developed immune system in human individuals and the relatively low frequency of immune stimulating side effects.
Predicition of systemically applied compounds
There is an urgent need to improve the prediction of immune-mediated side effects of drugs, biological agents and chemicals. Available animal and in vitro tests (e.g. skin sensitizations and lymph node assays) are mainly positive with haptens or prohaptens[*], which rapidly become haptens. Nevertheless, these tests lack a reliable prediction of generalized forms of drug hypersensitivity as suggested with e.g. gemifloxacin (3). Furthermore, immune-mediated side effects appear only in a minority of patients which might have a special genetic predisposition (1) which is not present in animal models. Finally, new concepts of drug hypersensitivity, such as the presented p-i concept, are hardly covered by animal experiments or in vitro studies using animal cells (4).
For the safety assessment of chemicals or compounds which are applied topically and sensitize via skin or lung, in vitro tests seem to be promising (focused mainly on haptens or prohaptens and the pathway via dendritic cells). For testing the safety of systematically (orally or parentally) applied drugs, in vitro tests has to be developed and require human material.
The p-i concept
This concept includes the hypothesis that drugs can bind to the TCR* like to other receptors and that this interaction might stimulate T cells directly. It does not require a previous hapten-carrier formation (Fig. 1). The consequence is a reaction mimicking an immune reaction, but triggered by a drug. It does not (!) require the generation of an own immune response to the hapten (as the drug is no hapten) or the involvement of the innate immune system. This additional (alternative) pathway is called pharmacological interaction with immune receptors (p-i concept) (5). Considering this pathway might be highly relevant for detecting the potential hypersensitive (allergic) activity of a given compound and provides a good explanation for a number of open questions in drug hypersensitivity reactions (5).
Initiating an immune response in vitro
The p-i concept was originally established based on comparing the immune response of patients allergic to sulfamethoxazole (SMX) to the parent, non-haptenlike compound and to its main metabolite, the hapten Sulfamethoxazole-Nitroso (SMX-NO). SMX-NO binds covalently to proteins or peptides.
Hapten-like drugs and chemicals can easily induce an in vitro immune response by culturing peripheral blood mononuclear cells (PBMC) of nonsensitized individuals with the hapten for a prolonged time period. We could confirm this finding (6) and tested ten healthy nonsensitized individuals and found nine responders to SMX-NO.
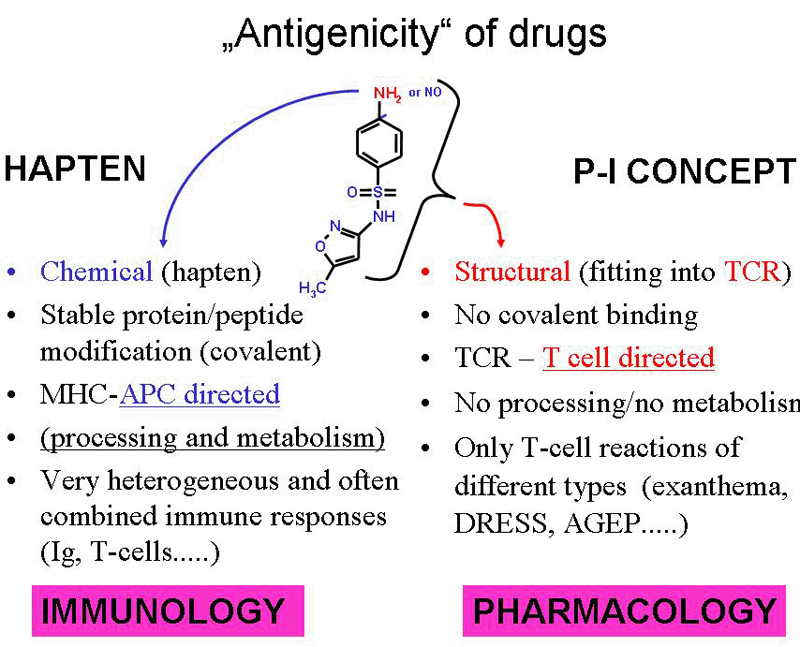
Fig. 1: Drugs gain antigenicity by two pathways.
A) they can bind covalently as haptens to peptides and proteins, modify them and make them immunogenic. This pathway is APC directed and determined by the chemical reactivity of a drug.
B) the p-i concept relies on the fitting of the drug into a certain T cell receptor for an antigen which happens to have a stimulating effect and leads to T cell expansion (see ref. 5).
Initiating an immune response via the p-i pathway
But what happens if not the hapten SMX-NO, but the parent compound SMX is added to the cell culture? According to the p-i-concept, T cells are the main target cells for the reaction (instead of dendritic cells). We were able to show that the metabolism of SMX to SMX-NO does not occur in vitro using peripheral blood mononuclear cells (PBMC) (7). The addition of SMX to PBMC of non sensitized individuals (previously never exposed to SMX) did not induce a proliferative response in PBMCs. However, by extending the cell culture period to 4-6 weeks and the repetitive addition of PBMCs as antigen presenting cells (APC) and IL2, we could detect some reactivity in three of ten healthy non-sensitized volunteers. The immune response was clearly directed to the non-reactive parent compound (SMX) itself and not to SMX-NO (6). One of these volunteers was tested several times, always giving the same positive response. We even cloned SMX-reactive T cells from this non-sensitized individual, which were specific for SMX (but not for SMX-NO) and had the same characteristics as SMX-specific T cells obtained from SMX-sensitized individuals. Thus, under certain culture conditions, SMX stimulates T cells via their TCR. However, only certain individuals seem to react in this assay, and a massive co-stimulation of the T cells seems to be required (6).
Long lasting immunological memory
It is well known that a previous drug hypersensitivity reaction poses a risk for a new one. This implies the existence of an immunological memory. But how many cells are actually involved? By analyzing the precursor frequency to five different drugs in five different patients with different forms of drug hypersensitivity reactions (sulfamethoxazole, carbamazepine, phenytoin, vancomycin and amoxicillin) (8) we were able to detect specific T cells in patients which had reacted 12 years to 4 months before the analysis was performed.
High precursor frequency of drug-specific T cells
The frequency of drug-reactive T cells was measured with two assays and compared to the frequency of T cells reactive with tetanus toxoid, which is a common recall antigen in Switzerland since the whole population is regularly vaccinated. CSFE labeling of peripheral blood lymphocytes allowed to measure the proliferation, as this CSFE fluorescein cell stain is halved at each cell division, allowing a precise calculation of how many cells have divided in a certain time period. In the ELISPOT analysis the cytokine production of drug-reactive T cells was determined after 36 hours of cell culture with the drug or tetanus. Both analysis gave quite similar values: a high frequency of drug-specific T cells were found in individuals with an allergy to the corresponding drug whereby the analysis was always negative to other drugs to which the patients had been exposed but not sensitized. The frequency was actually higher than the simultaneously measured tetanus response, as 1:250 to 1:10.000 of CD4+ T cells reacted with the different drugs. This detailed analysis of drug precursor frequencies is a good basis to establish tests to detect such cells for the in vitro diagnosis of delayed drug hypersensitivity reactions (see ref. 8). Drugs which stimulated via hapten as well as p-i mechanism induced quite a high precursor frequency, which reflects a vigorous cellular immune response.
From an individual (clinical) to a predictive test
The present data shows the ability to stimulate T cells pharmacologically via the T cell antigen receptor (p-i concept). This stimulation of T cells of non-sensitized individuals required repetitive stimulations and was strong enough to cause the T cells to divide and to expand. This drug-mediated stimulation via T cell receptors is per se a quite astonishing finding as it underlines the high potency of certain drugs to stimulate the immune system.
Clinical data indicates that the p-i pathway is probably more relevant than the hapten concept in eliciting generalized drug hypersensitivity reactions, while contact dermatitis is more due to the hapten mechanism.
In order to develop a predictive test based on the p-i concept and suitable for preclinical testing, several technical problems remain to be solved: The test must become far simpler, more robust and needs to be standardized. The human cells used for the in vitro tests must be carefully characterized. But the most important aspect is a better understanding of systemic drug hypersensitivity reactions in general. These questions are: What is the relationship of T cell stimulation to the clinical picture and why do only some individuals react - both in vivo as well as in vitro: is it due to the T cell receptor repertoire, immune regulation, genetic background or is it, as some data would indicate, a combination of all? With this knowledge, one could create sophisticated in vitro models with human cells which might even replace a substantial number of animal tests for the detection of hypersensitivity inducing drugs.
Published updated version of this Bulletin 33/2007 (PDF)
References:
- Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004; 428:486.
- Bala S, Weaver J, Hastings KL. Clinical relevance of preclinical testing for allergic side effects. Toxicology 2005; 209:195-200.
- Schmid DA, Campi P, Pichler WJ. Hypersensitivity Reactions to Quinolones, Current Pharmaceutical Design, in press, 2006
- Pichler WJ. Predictive drug allergy testing: an alternative viewpoint. Toxicology 2001; 158:31-41.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med 2003; 139:683-93.
- Engler OB, Strasser I, Naisbitt DJ, Cerny A, Pichler WJ. A chemically inert drug can stimulate T cells in vitro by their T cell receptor in non-sensitised individuals. Toxicology 2004; 197:47-56.
- Burkhart C, von Greyerz S, Depta JP, Naisbitt DJ, Britschgi M, Park KB, et al. Influence of reduced glutathione on the proliferative response of sulfamethoxazole-specific and sulfamethoxazole-metabolite-specific human CD4+ T-cells. Br J Pharmacol 2001; 132:623-30.
- Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol 2006;117:455-62.
| [*] | T cell immune responseCertain chemicals (including biological agents and drugs) are immunotoxic, which means they can stimulate or suppress the human immune system. The adverse side-effects of a stimulated immune system can be sensitization or autoimmunity with an inflammatory reaction. In rather rare cases, sensitized individuals can experience an allergic reaction to a second exposure of the stimulus (antigen, allergen) and become hypersensitive. Well known are compounds which, when given on the skin, cause inflammation of skin areas (allergic contact dermatitis). The side effects can be even more severe when an immune stimulant enters the body and reaches the blood system (e.g. after oral ingestion) inducing a T cell-mediated immune response. T cells are a subpopulation of lymphocytes and are involved in most allergic reactions (hypersensitivity). T cells are able to recognize peptides, lipids, metals and complexes consisting of chemicals and peptides as antigens. The immunogenicity of chemicals (but not of biological agents = proteins) is generated by the previous covalent binding of the hapten to a protein, which is then processed to a peptide and presented on MHC-structures (= HLA-molecules). Such hapten-peptide complexes are recognized by some T cells with the fitting T cell receptors (TCR) for the particular antigen. However, W.J. Pichler argues that this hapten model is not a sufficient explanation for many drug-induced side effects. He and his group showed that a direct non-covalent binding of drugs to the TCR is possible and under certain circumstances stimulatory for T cells (p-i concept). Binding of the drug to the TCR activates T cells, whereby MHC interaction (regardless of the enclosed peptide!) with the TCR supports this drug mediated signal. The T cells start to divide and organize an inflammatory response in the body by secreting cytokines and killing other cells. These immune-mediated inflammatory responses may cause mild symptoms such as maculopapular exanthema, but a substantial fraction of these reactions are severe, causing Stevens-Johnson-Syndrome, toxic epidermal necrolysis, hepatitis, pancreatitis, fever, vasculitis, eosinophilia, and even death. |
| Last modified 2009/06/13 |