
In 2011 the Foundation provided a total of Fr. 660,606 for 18 research projects. The Confederation and Interpharma made a total contribution of Fr. 770,000. The Administrative Board approved 6 new projects, while 11 projects were successfully completed; 28 applications were rejected. The 3R-Info-Bulletins 45 - 47, which were circulated to around 1,000 readers, included the results of three of the completed projects. The members of the Foundation’s official bodies were re-elected by the Administrative Board for a further period of office of four years. The deed of foundation and the regulations were revised in order to bring them into line with current requirements. To mark its 25th anniversary in 2012, the Foundation plans to organise a scientific workshop together with the Swiss Laboratory Animal Science Association, and ecopa (European Consensus Platform for 3R Alternatives to Animal Experimentation) will be invited to hold its annual general meeting at the same time and in the same location. The future direction of the Foundation’s activities was the focus of debate among the Administrative Board. A decision will be based on the findings of a study of the practical impact of the results of funding research projects. The outcome of this study will help the Administrative Board adopt a future strategy for the Foundation.
The 3Rs are Replace, Reduce and Refine animal experimentation. The 3Rs must be the guiding principles behind animal experimentation; if a study can be carried out without using any laboratory animals then such a procedure must be used. If it is essential to use laboratory animals under the terms of animal protection legislation the number used must be kept to a strict minimum. The third “R” requires that animals used for laboratory experiments be made to suffer an absolute minimum of pain and/or stress. The 3R Research Foundation funds research projects whose aim is to improve present-day experimental methods from the point of view of the 3Rs.
Detailed information about all the Foundation’s activities can be found on its website at www.forschung3r.ch. Apart from an overview of the projects funded, the section entitled “3R-Methods“ includes selected 3R methods intended to resolve specific problems in the field of life sciences.
A total amount of Fr. 660,606.00 was paid out for 13 ongoing projects and 5 that were completed during 2011.
Six new projects were approved in 2011 for which a total of Fr. 686,020.00 was earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Nerve-cell mimicking liposomes as an in vitro alternative for demonstrating the potency of toxins with multistep pathways such as Botulinum neurotoxins (BoNT) (125/11) Dr. Oliver G. Weingart, Institute of Food Sciences, Nutrition and Health, Zurich Federal Institute of Technology, Switzerland. The registration authorities demand that the level of potency be determined for each lot of toxins manufactured from living organisms before they are accepted for medical use. Until now, this has been done using the extremely painful LD50 test on mice. An alternative method involves using in part cell systems, but they are in many cases not sufficiently sensitive as well as being difficult to reproduce, or else the protocols are not published as they form part of the production procedure for the toxins. Using liposomes into which the individual steps in the potency pathway have been introduced should make up for this deficiency in the cell systems. It would then be possible to determine the potency level without using the LD50 test in mice.
Model development and validation to investigate myeloid cell homeostasis (126/11) Dr. Charaf Benarafa, Theodor Kocher Institute, University of Berne, Switzerland. Neutrophil granulocytes play an important role in inflammatory diseases and in the body’s defence against pathogens. They are found only in minute quantities in the blood. In order to investigate their function a large number of laboratory mice, including transgenic types, have normally been used until now. Through the genetic manipulation (Hoxb8) of neutrophil precursor cells the research team intend to obtain functioning neutrophil granulocytes from the precursor cells. These cells will need to be characterised in order to determine whether they are indeed behaving in the same way as differentiated cells. If the project is successful it will no longer be necessary to use a large number of laboratory mice nor to breed various types for laboratory experiments.
Establishing a novel system for quantitative production of murine basophils in vitro (127/11) Prof. Thomas Kaufmann, Institute of Pharmacology, University of Berne, Switzerland. Basophil blood cells fulfil non-redundant immunoregulatory and pro-inflammatory functions, despite constituting only 1% of all cells in the blood. It is not possible to culture such cells in vitro. For this reason, a large number of laboratory animals are required for the simplest functional or biochemical experiments using mouse models. Prof. Kaufmann intends to insert a genetic sequence (Hoxb8) into basophil precursor cells in mouse bone marrow in order to immortalise the cells and thus make it possible to culture them in vitro. Subsequently, the cells will be made to differentiate into mature basophils (using 4-hydroxytamoxifen). These cells can then be used for immunology testing (e.g. in transfection experiments). In this way it will be possible to replace a large number of mice, genetically modified mice and other animals required for their production.
Genetic modification of the human airway epithelium – a paradigmatic system to study host responses to human respiratory viruses (128/11) Dr. Volker Thiel, Institute of Immunobiology, St. Gallen Cantonal Hospital, Switzerland. The human airway epithelium is the point of access for many respiratory viruses, e.g. influenza viruses. In this project human airway epithelial cells acquired by bronchoscopy will be cultured instead of resorting to the frequently used rodent models (to determine mechanisms of infection). The biopsies contain various cell types that are typical of the epithelium, including cells and cilia that produce mucus. In order to determine the mechanisms (epithelium-virus interaction) the cultured cells will be genetically modified using established transduction methods (reporter proteins). As part of the project, the genetic modification methods will be further developed and standardised. This will allow the influence of certain proteins on virus infection rates to be examined in vitro. This procedure will not only lead to the replacement of test animals as well as those animals used in the rodent models but also enable the researchers to verify the validity of the results obtained using these animal models in relation to humans.
Using a microfluidic chamber to study mitochondrial transport in PTEN and SOCS3 dependent axonal regeneration (129/11) Prof. Zhigang He, Children’s Hospital, Boston, USA. The impaired regeneration of nerve cells in the central nervous system following damage to the spinal cord will be investigated in depth using, among other things, transgenic mouse models and will be verified in primates. Mechanistic investigations require the use of a large number of mice of many differently genetically modified strains and cause a great deal of suffering to the laboratory animals. Various therapies are being investigated at present. In this project a two-part microfluidic chamber will be used to study how axonal regeneration is inhibited. Cortical neurones that have been extracted from genetically modified mice will be seeded and, thanks to the special design of the chamber, axonal regeneration can be studied. This process will enable the researchers to identify new genes and structures that are involved in axonal regeneration. Potential pharmacological products will be tested. It should be possible to reduce the number of mice required for the in vivo testing, involving considerable suffering, by up to 50%.
Establishment of an in-vitro organ-slice defect model for meniscal repair in orthopaedic research (130/11) Prof. Ernst B. Hunziker, Center of Regenerative Medicine for Skeletal Tissues, University of Berne, Switzerland. Meniscus injuries are common and are caused by sport, among other things, or by osteoarthritis or rheumatoid arthritis in overweight patients. Sheep and goats are commonly used to test new treatments, such as replacing the damaged meniscus with synthetic material or even a prosthesis. In this project, Professor Hunziker and his team will be testing new methods in a specially developed chamber in which fine meniscus slices from cow joints obtained from slaughter-houses will be cultured. Investigation processes and incubation conditions will be improved (growth factors, microgranules, material used for the joint mucus membrane, etc.) until an optimum therapeutic concept, i.e. the repair of the meniscus lesion, is obtained. This method allows for the entire repair process to be followed for up to 6 weeks, thus obviating the need for up to 80% of laboratory animals in which a meniscus lesion has been artifically induced.
Magnetic resonance imaging (MRI) for the non-invasive assessment of lung inflammation and pulmonary function in the rat (82/02) Dr. Nicolau Beckmann, Novartis Institute of Biomedical Research, Basle, Switzerland. To develop treatments for asthma, induced inflammatory and fibrotic changes (early stages of asthma) have been characterised in rats using magnetic resonance imaging (MRI). Compared with conventional pulmonary function measurement and terminal histopathological analyses, the use of this non-invasive method means that the number of laboratory animals required can be reduced by up to 90%, and the condition of the individual animals can be monitored closely. From the point of view of animal welfare, this is a decisive advantage since, if the medication being tested has no effect, individual animals can be withdrawn from the experiment in the early stages of asthma.
Non-mammalian experimental models for the study of bacterial infections (NEMO network) (99/05) Prof. Pierre Cosson, UMC, Faculty of Medicine, Department of Cellular Physiology and Metabolism, Geneva, Switzerland. The mechanisms involved in bacterial infections and the cellular defence strategies can also be studied using amoeba and the fruit fly (Drosophila). With this specialised knowledge it would be possible to carry out certain infection experiments using amoeba and fruit flies instead of laboratory animals. In order to promote the use of this method, funding was provided for a 3-year platform for 5 research groups on the 3R Foundation’s website. It is to be hoped that other researchers will use the available models with amoeba and fruit flies for screening potentially effective antibiotics and thus reduce the number of conventional infection studies carried out using rodents.
Organotypic CNS slice cultures as an in-vitro model for immune mediated tissue damage and repair in multiple sclerosis (101/06) Prof. Norbert Goebels, Neuroimmunology Unit, Neurological Clinic, University Hospital, Zurich, Switzerland; at present at the Heinrich-Heine University, Department of Neurobiology, Düsseldorf, Germany. The processes that lead to lesions in multiple sclerosis are often studied using an animal model, namely the EAE mouse model. As a part replacement, Prof. Goebels’ research team examined the immunological mechanisms of brain damage in cultured brain tissue slices of transgenic mice. They were able to demonstrate that antibodies and a complementary system cause demyelisation of the axons without damaging them. Antigen-specific cytotoxic (CD8) T-cells did cause collateral damage to the axons, however, but only when the tissue and the cytotoxic T-cells had been activated beforehand. To date it has not been possible to demonstrate this type of damage using the EAE animal model. This could result in a fall in popularity of the animal model and consequently a reduction in the number of EAE experiments carried out.
An in vitro screening assay for stem/progenitor cells in organotypic hippocampal slice cultures: validation in an experimental model of infant rat pneumococcal meningitis (103/06) Prof. Stephen Leib, Institute for Infectious Diseases, University of Berne, Switzerland. Cell changes were studied in organotypic hippocampal slice cultures in cell types that might play a role in brain damage and regeneration following bacterial meningitis. The research team succeeded in differentiating the stage of development of neuronal stem/progenitor cells cultured over a period of weeks. The suitability of such cells for transplantation and their behaviour in neuronal tissue were also studied. It was possible to show that the cells interact from a functional point of view with those in the hippocampus tissue slice. This in vitro method could be used for the relevant preliminary investigations and laboratory animals would only be required for the final confirmation of the in vitro findings.
Evaluation of an in vitro model to identify host parameters associated with virulence of Toxoplasma gondii strains (107/07) Dr. Sushila D’Souza, Pasteur Institute, Brussels, now IPH, Laboratory of Toxoplasmosis, Brussels, Belgium. Toxoplasmosis, a disease which can be acute in man, is caused by Toxoplasma gondii (natural host: cats). The virulence of samples contaminated with Toxoplasma gondii is normally tested on mice. To replace these tests, which cause extreme suffering in the laboratory animals, Dr. D’Souza’s research team have cultured parasites from strains with various known levels of virulence together with human intestinal cells. A correlation between the level of virulence and the number and extent of the foci formation on the cell monolayer was observed, while a reverse correlation was seen with the degree of inhibition of the β-defensin2 proteins, which are responsible for defence against toxoplasmosis, in the intestinal cells. These changes could be used as an indicator for determining the virulence of Toxoplasma gondii without using laboratory animals.
In vitro fish hepatocytes as a source of metabolic clearance data in alternative approaches for the reduction or replacement of in vivo bioaccumulation testing with fish (108/07) Prof. Helmut Segner, Centre for Fish and Wildlife Health, University of Berne, Switzerland. It is necessary to determine the possible concentration in fish of chemical substances with a certain lipophilic character that enter our environment in large quantities. In order to obtain this information without studying the fish themselves it is necessary to examine the possibility of metabolic conversion, since this influences the concentration of such substances in the living organism. Using reference substances it has been possible to demonstrate this metabolic conversion in freshly extracted fish liver cells. By standardising in vitro methods the validity of non-animal testing has been improved to such a degree that the results are entirely comparable to those obtained using live fish.
Preparation and evaluation of lipoprotein fractions for the replacement of fetal bovine serum in cell culture media (109/08) Prof. Paul Honegger, Department of Physiology, University of Lausanne, Switzerland. In most cases cell cultures require media that include fetal bovine serum in order to achieve optimal growth and to maintain cell function. Since the composition of the serum is not defined and varies from charge to charge, and since the acquisition of the serum from unborn calves should be avoided from an animal protection point of view, researchers have long been trying to find a defined serum replacement. Prof. Honegger’s research team have succeeded in demonstrating that, contrary to expectations, a macromolecular protein is not responsible for the stabilisation of cell function in the lipoprotein fraction.
Development of an in vitro assay for screening of antischistosomal drugs (110/08) Prof. Jennifer Keiser, Swiss Tropical and Public Health Institute, Basle, Switzerland. Infection by the parasite Schistosoma, which causes bilharziasis, can be treated in humans by targeting juvenile or adult Schistosoma parasites (in blood or organs respectively). At present, the parasites are obtained using mice or hamsters and the efficacy of potential treatments is tested in mice that have been infected with the parasite. Unlike the adult stages, the juvenile form can be cultured in vitro. The studies carried out by Prof. Keiser have shown that substances that were not effective against the juvenile stages of the parasite also showed no effect on the adult stages. Through improved in vitro testing it will no longer be necessary to carry out subsequent tests on laboratory animals.
Establishment of an organ ex-vivo tissue slice model for cardiovascular research, in particular for therapeutic atherosclerosis targeting (111/08) Prof. Patrick Hunziker and Dr. Xueya Wang, Clinic for Intensive Medicine, University of Basle, Switzerland. In this project, aorta tissue of transgenic (ApoE-/-) mice was extracted and cultured ex vivo. A method was developed for obtaining the tissue and its subsequent on-line observation under a fluorescence microscope. It was possible to identify and characterise the sclerotic sections of the walls of the aorta (plaques) following perfusion of the aorta with specific markers. The time taken for changes to the cells could also be determined. The results obtained corresponded to a great extent to findings from studies using ApoE-/- mice, which shows that many studies, for example for preselecting potential new medicines, could be carried out ex vivo.
A novel in vitro model for holistic assessment and optimisation of engineered tissue for functional cartilage repair (112/08) Dr. Zhijie Luo, Leeds Dental Institute, Leeds, UK. In the future the use of implanted, synthetic cartilage (substrate and chondrocytes) could allow comprehensive healing in the case of osteoarthritis. The reaction between this engineered tissue and the healthy cartilage is at present being studied in an intact organism, defects first being induced in the cartilage. These experiments cause considerable suffering in the laboratory animals used, mainly rabbits and goats but also sheep, pigs and dogs. As part of this project a method has been developed whereby the interaction between the existing and the implanted tissue can be measured. A new type of bioreactor has been further developed to enable cartilage rings, including test filling, to be cultured over a number of weeks and under cyclic compression. In this project, the bioreactor has been optimised, the influence of cyclic compression on cartilage repair has been studied and two different types of substrate for the cartilage cells have been tested. Establishing functional and well characterised bioreactors for studying cartilage defects and their repair is highly relevant to the 3R principles since there is at present intensive research in this area (keyword: ageing population). The outcome is that painful experiments using laboratory animals can be avoided.
Reducing the number of fish and their suffering during acute toxicity testing of potential environmental pollutants (OECD Guideline no. 203) (114/08) Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland. Through this project it has been possible to provide practical suggestions as to how the fish test involved in ecotoxicological studies (OECD guideline no. 203) can be improved from a 3R point of view. Thanks to retrospective analyses of hundreds of data series from fish tests and a mathematical simulation, it has been possible to demonstrate that the number of fish used per test group could be reduced by 14% without any loss of quality in the results. A further reduction in the number of fish required can be achieved if the fish embryo test is used to determine the initial dose. The results obtained by Dr. Rufli have been discussed and accepted by selected experts in Europe and the USA. Since fish are caused level 3 suffering if the required maximum dose is used (and is effective), an attempt is being made to instigate a request from an OECD country (e.g. Switzerland) for a change in the OECD guidelines worldwide.
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
Serum-free defined media, a largely unsolved problem in cell culture (no. 45, February 2011) In most cases cell cultures require media that include fetal bovine serum in order to achieve optimal growth and to maintain cell function. Since the composition of the serum is not defined and varies from charge to charge, and since the acquisition of the serum from unborn calves should be avoided from an animal protection point of view, researchers have long been trying to find a defined serum replacement. Prof. Honegger’s research team, Department of Physiology, University of Lausanne, Switzerland, have succeeded in demonstrating that, contrary to expectations, a macromolecular protein is not responsible for the stabilisation of cell function in the lipoprotein fraction.
Toxoplasma gondii virulence is predictable in cultured human cells (no. 46, May 2011) Toxoplasmosis, a disease which can be acute in man, is caused by Toxoplasma gondii (natural host: cats). The virulence of samples contaminated with Toxoplasma gondii is normally tested on mice. To replace these tests, which cause extreme suffering in the laboratory animals, Dr. D’Souza’s research team, Pasteur Institute, Brussels, Belgium, have cultured parasites from strains with various known levels of virulence together with human intestinal cells. A correlation between the level of virulence and the number and extent of the foci formation on the cell monolayer was observed, while a reverse correlation was seen with the degree of inhibition of the ?-defensin2 proteins, which are responsible for defence against toxoplasmosis, in the intestinal cells. These changes could be used as an indicator for determining the virulence of Toxoplasma gondii without using laboratory animals.
Metabolism as part of alternative testing strategies in fish (no. 47, October 2011). It is important to be able to determine the possible concentration in fish of lipophilic contaminants that are released into the environment. In order to obtain such information without examining the fish themselves, it is necessary to determine to what extent such substances are metabolised, since this would influence their concentration in the living organism. Prof. Segner’s research team at the Centre for Fish and Wildlife Health of the University of Berne in Switzerland was able to demonstrate this metabolic processing in freshly isolated fish liver cells using reference substances. By standardising in vitro reports it has been possible to improve the validity of the results obtained using this animal-free method to such a degree that they are comparable with values obtained in fish.
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (association of pharmaceutical companies that carry out research, comprising at present Actelion Ltd, Merck Serono Ltd, Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, and the associated members Bayer (Switzerland) Ltd, Cilag Ltd and Vifor Ltd) and the Animalfree Research Foundation (animal protection). The Foundation was entered in the commercial register on 18 August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods through grants for research projects as well as to implement and promote the 3R principles. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer improvements vis-à-vis standard animal experimentation in line with the 3R motto, Replace, Reduce, Refine.
A broad range of projects is funded on the condition that they are likely to replace animal experimentation or to reduce the number of animals used or the stress and/or pain suffered. Accordingly, projects based on the Foundation’s three principles and covering any of a broad selection of bio-medical disciplines will be taken into consideration.
The Administrative Board of the Foundation is made up of nine members, two representing the Swiss parliament (1 seat vacant), two representing animal protection, two from Interpharma and two from the Federal Veterinary Office, as well as a representative of other interested circles. Current members are:
Christine Egerszegi, member of the Council of States, Mellingen (Chairwoman)
Dr. Peter Bossard, Animalfree Research Foundation, Zurich (Vice-Chairman)
Dr. Franz P. Gruber, Doerenkamp-Zbinden Foundation, Küsnacht
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld
Silvia Matile-Steiner, lawyer, Reinach
Dr. Markus Schmutz, Novartis Pharma AG, Basle (as from 30.3.2011)
Nathalie Stieger, economist, F. Hoffmann-La Roche Ltd., Basle (as from 1.1.2012)
Prof. Hans Wyss, Director of the Federal Veterinary Office, Berne-Liebefeld
Prof. Peter Maier, University of Zurich (Chairman)
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Prof. Clemens A. Dahinden, Institute of Immunology and Allergology, University Hospital, Berne
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne
Prof. Andrew Hemphill, Institute of Parasitology, University of Berne
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld
Dr. Kurt Lingenhöhl, Novartis Pharma Ltd, Basle
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld (until 1.6.2010)
Dr. Martin Reist, Veterinary Public Health Institute, University of Berne
Dr. Stefanie Schindler, Animalfree Research Foundation, Zurich
Prof. Peter Maier, University of Zurich
Ernst P. Diener, lawyer, Münsingen
Waber Treuhand GmbH, Einigen
Federal Department of Home Affairs
The Foundation’s official bodies (the Administrative Board and the Evaluation Committee) and the Administrator were re-elected for a further period of office from 2011 to 2014. New additions to the Administrative Board are Dr. Markus Schmutz from Novartis Pharma in Basle and Nathalie Stieger, a graduate in economics from St. Gallen University, who works at F. Hoffman-La Roche also in Basle.
In the Foundation’s twenty-fifth year of existence the Administrative Board met twice, namely in March and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2010, the Board addressed the following issues.
At its meeting in March, the Board focused on the financial statements for 2010 and the re-election of members of the Foundation’s official bodies for the period 2011-2014, as well as updating the deed of foundation and totally revising the regulations. Furthermore, research funds were earmarked for ongoing projects, 2 new projects were approved and 9 completed projects were received. The updating of the deed of foundation centered on extending the aims and purposes to include “the implementation and promotion of the 3R principles”, the adoption of the Evaluation Committee as an official body of the Foundation and the revision of the appeal process. The modification of the deed of foundation was approved by the supervisory body on 28 September 2011. The changes made to this deed were implemented in the regulations concerning internal procedures and those relating to the functioning of the Foundation’s official bodies were updated to meet current requirements.
At the December Board meeting, the focus was on financial issues. Owing to the fact that the necessary funds are not forthcoming, it is not possible to approve all the applications recommended and considered worthy of funding by the Evaluation Committee. It was decided to approve at least 4 applications; moreover in 2012 new applications for funding will be considered only in autumn in order to lessen the burden on the budget 2012. Project outlines are to be submitted in the spring. Applicants whose proposed projects are relevant to the 3R principles will then be asked by the Evaluation Committee to draw up a detailed application which will be approved or not by the Administrative Board in autumn. It was decided to call an extraordinary meeting of the Administrative Board at the beginning of 2012 to discuss in detail basic issues concerning the direction of the Foundation’s activities and funding. Finally, the Administrative Board approved the guidelines relating to the functioning of the Evaluation Committee and noted that the tax authorities have confirmed that, in view of the nature of its work, the Foundation is not liable for taxes under the terms of its modified deed of foundation. The focus then turned to discussion with members of the Evaluation Committee of the Foundation’s activities in 2011 and efforts in the direction of networking. The meeting was rounded off with a dinner.
The Strategy Committee set up by the Administrative Board held various meetings at which it drew up proposals for celebrating the Foundation’s 25th anniversary, as well as a basis for deciding on the focus of the Foundation’s activities in the future.
The Administrator is responsible for the day-to-day running of the Foundation; he deals with all matters that cannot be passed on to anyone else. In particular, he prepares all the necessary information for the Administrative Board to take their decisions, as well as dealing with correspondence with applicants and project managers. He also deals with payments, book-keeping, closing the books at the end of the financial year and the budget. In addition, he prepares the text of the Annual Report as well as texts for the Foundation’s website.
Under the chairmanship of the Scientific Adviser, the Evaluation Committee held two meetings during the year, where in particular they examined 34 new applications for funding and evaluated the 11 completed projects. In addition it drew up guidelines for how the Evaluation Committee operates. The voluntary work of the members of the Evaluation Committee in this connection is much appreciated.
The Scientific Adviser's tasks included publishing the 3R-Info-Bulletins (in the form of brochures and on the Foundation’s website at www.forschung3r.ch), writing the brief scientific reports in English which present the projects receiving funding on the Foundation’s website and regularly updating these reports. He was also kept busy – as always – advising applicants and project managers, obtaining intermediate reports, evaluating project outlines, dealing with enquiries and explaining why projects had been rejected. Finally, he represented the Foundation at several scientific meetings in Switzerland and abroad, namely at the 8th World Congress on Alternatives and Animal Use in the Life Sciences, organised in Montreal, Canada, and as a member of the Mirror Group of the EPAA Initiative in Brussels (for further information visit www.ecopa.eu and http://ec.europa.eu/enterprise/epaa/index_en.htm).

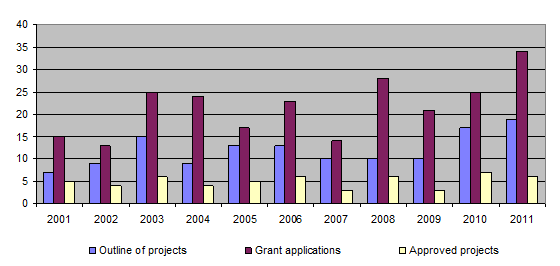
The bar-chart shows that over the past few years the number of project outlines and applications has increased considerably. In contrast, the number of projects approved has remained more or less unchanged, owing to the Foundation’s financial limitations. On average, around 5 projects have been approved for funding each year. The long-term approval rate for applications is only just 30%. Basically, this figure reflects the careful consideration given to each application from the point of view of its relevance to the 3R principles. The number of projects approved is always limited by the level of available funding.
During the year 11 projects were completed (82-02, 99-05, 101-06, 103-06, 107-07, 108-07, 109-08, 110-08, 111-08, 112-08, 114-08). Together with those projects completed earlier, this brings the total of finished projects to 113 out of 130.
| 130/11 | Prof. Ernst B. Hunziker Center of Regenerative Medicine for Skeletal Tissues, University of Berne, Switzerland Establishment of an in-vitro organ-slice defect model for meniscal repair in orthopaedic research |
| 129/11 | Prof. Zhigang He Children’s Hospital, Boston, USA Using microfluidic chamber to study mitochondrial transport in PTEN and SOCS3 dependent axonal regeneration |
| 128/11 | Dr. Volker Thiel Institute of Immunobiology, Cantonal Hospital St. Gallen, Switzerland Genetic modification of the human airway epithelium – a paradigmatic system to study host responses to human respiratory viruses |
| 127/11 | Prof. Thomas Kaufmann Institute of Pharmacology, University of Berne, Switzerland Establishing A Novel System For Quantitative Production of Murine Basophils In Vitro |
| 126/11 | Dr. Charaf Benarafa Theodor Kocher Institute, University of Berne, Switzerland Model development and validation to investigate myeloid cell homeostasis |
| 125/11 | Dr. Oliver G. Weingart Institute of Food Sciences, Nutrition and Health, Zurich Federal Institute of Technology, Switzerland Nerve-cell mimicking liposomes as an in vitro alternative for demonstrating the potency of toxins with multistep pathways such as Botulinum neurotoxins (BoNT) |
A complete list of projects with summaries of each can be found on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
The brief scientific project reports in English, which are updated once a year, indicate that almost all projects have progressed well. These reports published on the internet are much appreciated by those involved in the research projects as a platform for presenting their work. From the opposite point of view, this system also enables other researchers all over the world to discover new 3R methods without delay.
In 2011 three more new 3R-Info-Bulletins (ISSN 1421-6590) were published in English and distributed to some 1,000 interested parties. The information bulletins are also published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html), as well as in pdf format.
The latest 3R-Info bulletins are:
| No. | Date | Topic |
|---|---|---|
| 47 | October 2011 | Metabolism as part of alternative testing strategies in fish |
| 46 | June 2011 | Toxoplasma gondii virulence is predictable in cultured human cells |
| 45 | February 2011 | Serum-free defined media, a largely unsolved problem in cell culture |
A total of some Fr. 660,606 was paid out for research in 2011.
Operational expenditure for 2011 amounted to Fr. 215,137.13 (project monitoring and information Fr. 105,725.28, administrative costs including office infrastructure Fr. 109,411.85).
Total expenditure therefore amounted to Fr. 875,743.98.
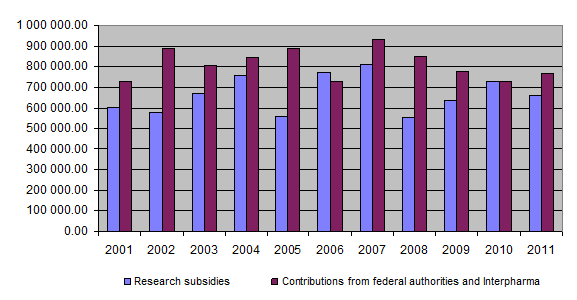
On the income side, the equal financial commitment of the federal authorities and Interpharma represented the basic funding for the Foundation’s activities. Accordingly, the federal authorities and Interpharma each granted the Foundation an amount of Fr. 365,000 in 2011. In addition, Interpharma provided an extraordinary donation of Fr. 40,000 in order to alleviate the Foundation’s financial problems somewhat. A further income item of Fr. 2,436.18 resulted from financial income and repayment of unused project funding.
Total income was therefore around Fr. 772,436.18 while total expenditure amounted to Fr. 875,743, giving an excess of expenditure over income of around Fr. 103,307. The unused funds item consequently fell from approximately Fr. 267,836 at the end of 2010 to Fr. 164,528 at the end of 2011, the latter constituting the Foundation’s new reserve of liquid assets.
At the end of 2011 the total earmarked for projects approved by the Board but not yet paid out amounted to Fr. 973,059. This future liability is covered by Interpharma’s promise of funding (V). The Foundation’s credit with this institution amounted to Fr. 1,197,000 at the end of 2011.
The budget for 2012 includes around Fr. 590,746 for current projects and a maximum amount of Fr. 500,000 for new projects.
At the end of 2011 a total of Fr. 17,763,938.81 had been granted for projects and other subsidies, of which Fr. 16,790,879.81 had been paid out so far. Together the federal authorities and Interpharma have contributed Fr. 19,946,000 to the Foundation since 1987.
Should the evident trend over the last three years persist, namely a rise in the number of applications worthy of support vis-à-vis unchanged contributions received from the Confederation and Interpharma, it is to be feared that in the future it will not be possible to fund a greater number of 3R-relevant projects.
| Profit and loss account 2011 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 365 000.00 | ||
| Contribution from Interpharma | 405 000.00 | ||
| Total contributions | 770 000.00 | ||
| Interest on bank account | 1301.54 | ||
| Other income | 1134.64 | ||
| Total income | 772 436.18 | ||
Expenditure | |||
| Research grants | 660 606.85 | ||
| Project supervision and information | 105 725.28 | ||
| Administrative expenses | 109 411.85 | ||
| Total expenditure | 875 743.98 | ||
| Excess expenditure over income | - 103 307.80 | ||
| 772 436.18 | |||
| Balance as per 31st December 2011 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 215 095.21 | ||
| Accounts payable | 211.15 | ||
| Accounting apportionment assets | 2 281.60 | ||
Liabilities | |||
| Accounting apportionment liabilities | 52 059.10 | ||
| Unused project funds | |||
| Carried forward 1. 1. 2011 | 267 836.66 | ||
| expend. over income | -103 307.80 | 164 528.86 | |
| Capital of the Foundation | 1 000.00 | ||
| 217 587.96 | 217 587.96 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 973 059.00. | ||
Münsingen, 29 February 2012
3R RESEARCH FOUNDATION
Chairwoman: signed C. Egerszegi
Secretary: signed E. Diener
Waber Treuhand GmbH in Einigen audited the financial statements for the year according to standards of limited auditing and did not find any indication that the accounts and statements do not correspond to current legislation or the principles and regulations of the Foundation.