 |
de | fr | en print view![]()
3R-Project 99-05
The NEMO network (Non-mammalian Experimental Models for the study of bacterial infections)
Coordinator of the network:
Pierre Cosson
Centre Médical Universitaire, Département de Physiologie Cellulaire et Métabolisme, 1 rue Michel Servet, 1211 Genève, Suisse
pierre.cosson@medecine.unige.ch
Keywords: bacteria; drosophila; protozoa: amoeba; infectious diseases; reduction; replacement; infectiosity
Duration: 4 years Project Completion: 2010
Background and Aim
To test the ability of a bacteria to cause a disease it is usually necessary to infect a mammalian host and allow the disease to progress. These experiments inflict significant suffering to the animals. Our general aim is to stimulate the emergence of a community of scientists using alternative non-mammalian hosts for the study of bacterial infections. Our common belief is that many experiments currently carried out using mammalian hosts could be advantageously replaced by the use of alternative non-mammalian hosts.
The NEMO network of laboratories was created in February 2005, initially as an informal gathering of research groups involved in similar subjects. Our specific goals are:
1-To organize an annual meeting on the theme of Non-mammalian hosts for the study of bacterial infections, in order to stimulate exchanges among research groups.
2-To strengthen our research in this field through a series of collaborative projects, for which we hope to find financial support.
3-To publicize the use of alternative non-mammalian hosts in the scientific community.
More about the activity of NEMO:
see:
Annual Report 2005
The five research groups that originally created the NEMO network are:
Pr. Dr. P. Cosson, Centre Médical Universitaire, Geneva, Switzerland
pierre.cosson@medecine.unige.ch
Project:
Extensive analysis of bacterial virulence in Dictyostelium
Dr. Marie-Odile Fauvarque, CEA-Grenoble, Département de Réponse et Dynamique Cellulaires, France
marie-odile.fauvarque@cea.fr
Project:
Bacterial virulence and innate immune response: Drosophila as a model system.
Dr. G. Greub, Microbiology Institute, Faculty of Biology and Medicine, University of Lausanne, Switzerland
gilbert.greub@chuv.ch
Project:
Free-living amoebae as a tool to study intracellular pathogens.
Prof. Dr. Hubert Hilbi, Institute of Microbiology, ETH Zürich, Switzerland
hilbi @ micro.biol.ethz.ch
Project:
Amoebae: a cellular pathogenesis model for the legionnaires´disease agent Legionella pneumophila
Dr. Thierry Soldati, Department of Biochemistry, University of Geneva, Switzerland
thierry.soldati@biochem.unige.ch
Project:
The Amoeba Dictyostelium as a model host for Mycobacterium marinum infection and persistence.
Method and Results
see publications cited in the Annual Reports
References
Publications from members of NEMO since 2005:
Cosson et al.:
1. Benghezal, M., Fauvarque, MO., Tournebize, R., Froquet, R., Marchetti, A., Bergeret, E., Lardy, B., Klein, G., Sansonetti, P., Charette, S.J., Cosson, P. 2006. Specific host genes required for the killing of Klebsiella bacteria by phagocytes. Cell. Microbiol. 8 :139-148.
2. Charette, S., Cornillon, S., Cosson, P. 2006. Identification of low frequency knockout mutants in Dictyostelium discoideum by single or double homologous recombination. J. Biotechnology 122 :1-4.
3. Cornillon, S., Gebbie, L., Benghezal, M., Nair, P., Keller, S., Wehrle-Haller, B., Charette, S.J., Brückert, F., Letourneur, F., Cosson, P. 2006. An adehsion molecule in free-living Dictyostelium amoebae with integrin beta features. EMBO Reports. In press
4. Benghezal, M., Adam, E., Lucas, A., Burn, C., Orchard, M.G., Deuschel, C., Valentino, E., Braillard, S., Paccaud, J.P., Cosson, P. 2007. Inhibitors of bacterial virulence identified in a surrogate host model. Cell. Microb. 9:1336-42.
5. Charette, S., Cosson, P. 2007. A CHS/Beige homologue is involved in biogenesis of Dictyostelium secretory lysosomes. J. Cell Sci. 120:2338-43.
6. Froquet, R., Cherix, N., Burr, S., Frey, J., Vilches, S., Tomas, J.M., Cosson, P. 2007. An alternative host model to evaluate Aeromonas virulence. Appl. Environmental Microb. 73: 5657-9.
7. Alibaud, L., Köhler, T., Coudray, A., Prigent-Combaret, C., Bergeret, E., Perrin, J., Benghezal, M., Reimmann, C., Gauthier, Y., van Delden, C., Attree, I., Fauvarque, M.O.,
Cosson, P. 2008. Pseudomonas aeruginosa virulence genes identified in a Dictyostelium host model. Cell. Microb. 10: 729-40.
8. Bergeret, E., Perrin, J., Williams, M., Grunwald, D., Engel, E., Thevenon, D., Taillebourg, E., Bruckert, F., Cosson, P., Fauvarque, M.O. 2008. TM9sf4 is required for Drosophila cellular immunity via cell adhesion and phagocytosis. J. Cell Sci. 121:3325-34.
9. Froquet, R., Lelong, E., Marchetti, A., Cosson, P. 2009. Dictyostelium discoideum: a model host to measure bacterial virulence. Nature Protocols. 4:25-30.
10. Vallet-Gely, I., Novikov, A., Augusto, L., Liehl, P., Bolbach, G., Péchy-Tarr, M., Cosson, P.,Keel, C., Caroff, M., Lemaitre, B. 2009. Hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, is linked to cyclic lipopeptide production. Appl. Environmental Microb. 76: 910-21.
Fauvarque et al.
1. Avet-Rochex A, Bergeret E, Attrée I, Meister M and Fauvarque M-O (2005). Suppression of Drosophila cellular immunity by directed expression of the ExoS toxin GAP domain of Pseudomonas aeruginosa Cell. Microbiology 7 :799-810
2. Alibaud L, Köhler T, Coudray A, Prigent-Combaret C, Bergeret E, Perrin J, Benghezal M, Reimman C, Gauthier Y, vanDelden C, Attree I, Fauvarque MO and Cosson P. Pseudomonas aeruginosa virulence gnees identified in a Dictyostelium host model. Cell Microbiology, in press
3. Avet-Rochex A, Perrin J, Bergeret E, Fauvarque MO. (2007) Rac2 is a major actor of Drosophila resistance to Pseudomonas aeruginosa acting in phagocytic cells. Genes Cells. 12:1193-204.
4. Benghezal, M., Fauvarque, MO., Tournebize, R., Froquet, R., Marchetti, A., Bergeret, E., Lardy, B., Klein, G., Sansonetti, P., Charrette, S.J., Cosson, P. (2006) Specific host genes required for killingbacteria by phagocytes. Cell Microbiology , 8: 139-148.
5. Avet-Rochex A, Bergeret E, Attrée I, Meister M and Fauvarque MO (2005). Suppression of Drosophila cellular immunity by directed expression of the ExoS toxin GAP domain of Pseudomonas aeruginosa Cell. Microbiology 7 :799-810.
6. Bergeret E, Perrin J, Williams M, Grunwald D, Engel E, Thevenon D, Taillebourg E, Bruckert F, Cosson P and Fauvarque MO (2008). TM9SF4 is required for Drosophila cellular immunity via cell adhesion and phagocytosis.. J. Cell Science, 15 : 3325-3234
7. Alibaud L, Köhler T, Coudray A, Prigent-Combaret C, Bergeret E, Perrin J, Benghezal M, Reimman C, Gauthier Y, vanDelden C, Attree I, Fauvarque MO and Cosson P.
(2008) Pseudomonas aeruginosa virulence genes identified in a Dyctiostelium host model. Cell microbiology, 10:729-740
Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, Tricoire H, Benaud C, Baudier J, Taillebourg E and Fauvarque MO. (2009). The Ubiquitin Specific Protease dUSP36/Scny targets IMD to prevent Drosophila immune signalling.Cell Host & Microbe, 6, 309-320
Greub et al.
1. G. Greub, H. Lepidi, C. Rovery, JP. Casalta, G. Habib, F. Collard, PE. Fournier, and D. Raoult. Usefulness of valve analysis for the diagnosis of infective endocarditis. Am J Med, 2005;118:230-238.
2. G. Greub, J.-L. Mege, J.P. Gorvel, D. Raoult, S. Meresse. Intracellular traffickying of Parachlamydia acanthamoebae within human macrophages. Cell Microbiol, 2005;7:581-589.
3. Thomas, V., Herrera-Rimann, K., Blanc, D., Greub, G. 2006. Biodiversity of amoebae and amoebae-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72 :2428-2438.
4. Casson, N., Medico, N., Bille, J., Greub, G. 2006. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung pneumocytes and lung fibroblasts. Microb. Infect. In press.
5. Baud, D., L. Regan, and G. Greub. 2008. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy
outcomes. Curr Opin Infect Dis 21:70-76.
6. Baud, D., V. Thomas, A. Arafa, L. Regan, and G. Greub. 2007. Waddlia chondrophila, a potential agent of human fetal death.
Emerg Infect Dis 13:1239-43.
7. Borel, N., S. Ruhl, N. Casson, C. Kaiser, A. Pospischil, and G. Greub. 2007. Parachlamydia spp. and related Chlamydia-like
organisms and bovine abortion. Emerg Infect Dis 13:1904-7.
8. Casson, N., R. Michel, K. D. Muller, J. D. Aubert, and G. Greub. 2008. Protochlamydia naegleriophila as etiologic agent of
pneumonia. Emerg Infect Dis 14:168-72.
9. Casson, N., K. M. Posfay-Barbe, A. Gervaix, and G. Greub. 2008. A new diagnostic real-time PCR for the specific detection
of Parachlamydia acanthamoebae DNA in clinical samples. J Clin Microbiol.
10. Goy, G., V. Thomas, K. Rimann, K. Jaton, G. Prod'hom, and G. Greub. 2007. The Neff strain of Acanthamoeba castellanii, a
tool for testing the virulence of Mycobacterium kansasii. Res Microbiol 158:393-7.
11. Thomas, V., N. Casson, and G. Greub. 2007. New Afipia and Bosea strains isolated from various water sources by amoebal
co-culture. Syst Appl Microbiol 30:572-9.
12. Thomas, V. , Loret,J.F.;Greub,G.;. 2008. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment
plant. Appl Env Microbiol, submitted.
13. Baud, D., G. Goy, S. Gerber, Y. Vial, P. Hohlfeld, and G. Greub. 2009. Evidence of maternal-fetal transmission of Parachlamydia acanthamoebae. Emerg Infect Dis 15:120-1.
14. Baud, D., L. Regan, and G. Greub. 2008. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis 21:70-76.
15. Baud, D., V. Thomas, A. Arafa, L. Regan, and G. Greub. 2007. Waddlia chondrophila, a potential agent of human fetal death. Emerg Infect Dis 13:1239-43.
16. Borel, N., S. Ruhl, N. Casson, C. Kaiser, A. Pospischil, and G. Greub. 2007. Parachlamydia spp. and related Chlamydia-like organisms and bovine abortion. Emerg Infect Dis 13:1904-7.
17. Casson, N., J. M. Entenza, N. Borel, A. Pospischil, and G. Greub. 2008. Murine model of pneumonia caused by Parachlamydia acanthamoebae. Microb Pathog 45:92-7.
18. Casson, N., R. Michel, K. D. Muller, J. D. Aubert, and G. Greub. 2008. Protochlamydia naegleriophila as etiologic agent of pneumonia. Emerg Infect Dis 14:168-72.
19. Casson, N., K. M. Posfay-Barbe, A. Gervaix, and G. Greub. 2008. A new diagnostic real-time PCR for the specific detection of Parachlamydia acanthamoebae DNA in clinical samples. J Clin Microbiol.
20. Corsaro, D., V. Feroldi, G. Saucedo, F. Ribas, J. F. Loret, and G. Greub. 2009. Novel Chlamydiales strains isolated from a water treatment plant. Env Microbiol.
21. Goy, G., A. Croxatto, and G. Greub. 2008. Waddlia chondrophila enters and multiplies within human macrophages. Microbes Infect 10:556-62.
22. Greub, G. 2009. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin Microbiol Infect 15:18-28.
23. Thomas, V., J. F. Loret, M. Jousset, and G. Greub. 2008. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol 10:2728-45.
24. Baud, D., G. Goy, S. Gerber, Y. Vial, P. Hohlfeld, and G. Greub. 2009. Evidence of maternal-fetal transmission of Parachlamydia acanthamoebae. Emerg Infect Dis 15:120-1.
25. Baud, D., L. Regan, and G. Greub. 2008. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis 21:70-76.
26. Baud, D., V. Thomas, A. Arafa, L. Regan, and G. Greub. 2007. Waddlia chondrophila, a potential agent of human fetal death. Emerg Infect Dis 13:1239-43.
27. Borel, N., S. Ruhl, N. Casson, C. Kaiser, A. Pospischil, and G. Greub. 2007. Parachlamydia spp. and related Chlamydia-like organisms and bovine abortion. Emerg Infect Dis 13:1904-7.
28. Casson, N., J. M. Entenza, N. Borel, A. Pospischil, and G. Greub. 2008. Murine model of pneumonia caused by Parachlamydia acanthamoebae. Microb Pathog 45:92-7.
29. Casson, N., R. Michel, K. D. Muller, J. D. Aubert, and G. Greub. 2008. Protochlamydia naegleriophila as etiologic agent of pneumonia. Emerg Infect Dis 14:168-72.
30. Casson, N., K. M. Posfay-Barbe, A. Gervaix, and G. Greub. 2008. A new diagnostic real-time PCR for the specific detection of Parachlamydia acanthamoebae DNA in clinical samples. J Clin Microbiol.
31. Corsaro, D., V. Feroldi, G. Saucedo, F. Ribas, J. F. Loret, and G. Greub. 2009. Novel Chlamydiales strains isolated from a water treatment plant. Env Microbiol.
32. Goy, G., A. Croxatto, and G. Greub. 2008. Waddlia chondrophila enters and multiplies within human macrophages. Microbes Infect 10:556-62.
33. Greub, G. 2009. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin Microbiol Infect 15:18-28.
34. Thomas, V., J. F. Loret, M. Jousset, and G. Greub. 2008. Biodiversity of amoebae and amoebaeresisting bacteria in a drinking water treatment plant. Environ Microbiol 10:2728-45.
35. Corsaro, D., V. Feroldi, G. Saucedo, F. Ribas, J. F. Loret, and G. Greub. 2009. Novel Chlamydiales strains isolated from a water treatment plant. Environ Microbiol 11:188-200.
36. Croxatto A and Greub G. Early intracellular trafficking of Waddlia chondrophila in human macrophages. Microbiology, in press
Hilbi et al:
1. Albers, U., Reus, K., Shuman, H.A. & Hilbi, H. 2005. The amoebae plate test implicates a paralogue of lpxB in the interaction of Legionella pneumophila with Acanthamoeba castellanii. Microbiol. 151 :167-182.
2. Mampel, J., Spirig, T., Weber, S., Haagensen, J.A.J., Molin, S., Hilbi, H. 2006. Planktonic replication is essential for biofilm formation of Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72 :2885-2895.
3. Weber, S., Ragaz, C., Reus, K., Nyfeler, Y., Hilbi, H. 2006. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLOS Pathogens 2 :e46.
4. Hilbi, H., Weber, S. S., Ragaz, C., Nyfeler, Y. & Urwyler, S. (2007) Environmental predators as models for bacterial pathogenesis. Env. Microbiol. 9: 563-575.
5. Tiaden, A., Spirig, T., Weber, S.S., Brüggemann, H., Bosshard, R., Buchrieser, C. & Hilbi, H. (2007) The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell. Microbiol. 9: 2903-2920.
6. Albers, U., Tiaden, A., Spirig, T., Al Alam, D., Goyert, S.M., Gangloff, S.C. & Hilbi, H. (2007) Expression of Legionella pneumophila paralogous lipid A biosynthesis genes under different growth conditions. Microbiology 153: 3817-3829.
7. Tiaden, A., Spirig, T., Carranza, P., Brüggemann, H., Riedel, K., Eberl, L., Buchrieser, C. & Hilbi, H. Synergistic contribution of Legionella pneumophila lqs genes to pathogen-host interactions. Submitted.
8. Schroeder, G. N. & Hilbi, H. (2008) Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion and death by type III secretion. Clin. Microbiol. Rev. 21: 134-156.
9. Spirig, T., Tiaden, A., Kiefer, P., Buchrieser, C., Vorholt, J. A. & Hilbi, H. (2008) The Legionella autoinducer synthase LqsA produces an -hydroxyketone signaling molecule. J. Biol. Chem. 283: 18113-18123.
10. Ragaz, C., Pietsch, H., Urwyler, S., Tiaden, A., Weber, S.S. & Hilbi, H. (2008) The Legionella pneumophila phosphoinositide-4 phosphate-binding type IV substrate SidC
recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell. Microbiol. 10: 2416-2433.
11. Tiaden, A., Spirig, T., Carranza, P., Brüggemann, H., Riedel, K., Eberl, L., Buchrieser, C. & Hilbi, H. (2008) Synergistic contribution of the Legionella pneumophila lqs genes to pathogen-host interactions. J. Bacteriol. 190: 7532-7547.
12. Urwyler, S., Nyfeler, Y., Ragaz, C., Lee, H., Müller, L., Aebersold, R. & Hilbi, H. (2009) Proteome analysis of Legionella vacuoles purified by magnetic immuno-separation reveals secretory and endosomal GTPases. Traffic 10: 76-87.
13. Hilbi, H. (2009) Pathogene Bakterien als Zellbiologen - Wie Legionellen Phosphoinositid- Lipide von Wirtszellen ausnützen. BIOforum 01/2009: 2-4.
14. Brombacher, E., Urwyler, S., Ragaz, C., Weber, S. S., Kami, K., Overduin, M. & Hilbi, H. (2009) Rab1 guanine nucleotide exchange factor SidM is a major PtdIns(4)P-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284: 4846–4856.
15. Weber, S. S., Ragaz, C. & Hilbi, H. (2009) The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell. Microbiol. 11: 442-460.
16. Urwyler, S., Nyfeler, Y., Ragaz, C., Lee, H., Müller, L., Aebersold, R. & Hilbi, H. (2009) Proteome analysis of Legionella vacuoles purified by magnetic immuno-separation reveals secretory and endosomal GTPases. Traffic 10: 76-87.
17. Hilbi, H. (2009) Pathogene Bakterien als Zellbiologen - Wie Legionellen Phosphoinositid- Lipide von Wirtszellen ausnützen. BIOforum 01/2009: 23-25.
18. Brombacher, E., Urwyler, S., Ragaz, C., Weber, S. S., Kami, K., Overduin, M. & Hilbi, H. (2009) Rab1 guanine nucleotide exchange factor SidM is a major PtdIns(4)P-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284: 4846–4856.
19. Weber, S. S., Ragaz, C. & Hilbi, H. (2009) The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell. Microbiol. 11: 442-460.
20. Weber, S. S., Ragaz, C. & Hilbi, H. (2009) Pathogen trafficking pathways and host phosphoinositide metabolism. Mol. Microbiol. 71: 1341-1352.
21. Urwyler, S., Brombacher, E. & Hilbi, H. (2009) Endosomal and secretory markers of the Legionella-containing vacuole. Commun. Integrat. Biol. 2: 107-109.
22. Hilbi, H. (2009) Bacterial jailbreak sounds cellular alarm: phagosome membrane remnants trigger signaling. Cell Host Microbe 6:102-4.
23. Urwyler, S., Finsel, I., Ragaz, C. & Hilbi, H. Isolation of Legionella-containing vacuoles by immuno-magnetic separation. Curr. Prot. Cell Biol. In press.
24. Tiaden, A., Spirig, T., Sahr, T., Wälti, M. A., Boucke, K., Buchrieser, C. & Hilbi, H. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Env. Microbiol. In press.
25. Tiaden, A., Spirig, T. & Hilbi, H. Bacterial gene regulation by α-hydroxyketone signaling. Trends in Microbiology. Submitted (invited review).
26. Hilbi, H., Jarraud, S., Hartland, E. & Buchrieser, C. Update on Legionnaires’ disease: pathogenesis, epidemiology, detection and control. Mol. Microbiol. Submitted (meetingreport).
Soldati et al:
1. Hagedorn, M., Neuhaus, E.N., Soldati, T. 2006. Optimised fixation and immunofluorescence protocols for Dictyostelium cells. Methods Mol. Biol. Dictyostelium discoideum Protocols edited by Eichinger and Rivero, Humana Press Totowa, NJ. Chapter 20:327-338.
2.Gotthardt, D., Dieckmann, R., Blancheteau, V., Kistler, C., Reichardt, F., Soldati, T. 2006. Preparation of intact, highly purified phagosomes from Dictyostelium. Methods. Mol. Biol. Dictyostelium discoideum Protocols edited by Eichinger and Rivero, Humana Press Totowa, NJ. Chapter 26:439-448.
3. Soldati, T., Schliwa. 2006. Powering membrane traffic in endocytosis and exocytosis. Nature Reviews in Molecular Cell Biology. In press.
4. Hagedorn, M., and Soldati, T. Nonlytic ejection of pathogenic mycobacteria from its host is crucial for cell-to-cell spreading. (2008) submitted
6. Dieckmann, R., Gopaldass, N., Escalera, C., and Soldati, T. Monitoring time-dependent maturation changes in purified phagosomes from Dictyostelium discoideum. (2007) Methods Mol. Biol. in press.
7. Pino, P., Foth, B.J., Kwok, L., Sheiner, L., Schepers, R., Soldati, T., and Soldati, D. Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii (2007) PLOS Pathogens. 3(8):e115.
8. Hagedorn, M., and Soldati, T. Flotillin and the RacH GTPase modulate intracellular immunity of Dictyostelium to Mycobacterium marinum infection. (2007) Cell Microbiol. 9, 2716-33.
9. Hagedorn, M., Kyle H. Rohde, David G. Russell, and Soldati, T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science (2009) in press
10. Dieckmann, R., Gopaldass, N., Escalera, C., and Soldati, T. Monitoring time-dependent maturation changes in purified phagosomes from Dictyostelium discoideum. Methods Mol. Biol. (2008) 445, 327-337.
11. Dieckmann, R., and Soldati, T. Phagosome proteomes unite! A virtual model of maturation as a tool to study pathogen-induced changes. In Intracellular Niches of Microbes: A Pathogens Guide Through the Host Cell, Ed Schaible, U.E., and Haas, A. John Wiley & Sons, Ltd, (2008) in press.
12 . Hagedorn, M., and Soldati, T. Mycobacterium marinum. In Intracellular Niches of Microbes: A Pathogens Guide Through the Host Cell, Ed Schaible, U.E., and Haas, A. John Wiley & Sons, Ltd, (2008) in press.
13. Cosson, P, and Soldati, T., Eat, kill or die: when amoeba meets bacteria. Invited review for Current Opinion Microbiol. (2008) 11, 271-276.
14. Hagedorn, M., Kyle H. Rohde, David G. Russell, and Soldati, T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science (2009) 323, 1729-1733.
15. Dieckmann, R., and Soldati, T. Phagosome proteomes unite! A virtual model of maturation as a tool to study pathogen-induced changes In Intracellular Niches of Microbes: A Pathogens Guide Through the Host Cell, Ed Schaible, U.E., and Haas, A. John Wiley & Sons, Ltd, (2009) in press.
16. Hagedorn, M., and Soldati, T. Mycobacterium marinum In Intracellular Niches of Microbes: A Pathogens Guide Through the Host Cell, Ed Schaible, U.E., and Haas, A. John Wiley & Sons, Ltd, (2009) in press.
Figure 1. To study infectious diseases, it is not always necessary to infect animals. Researchers in the NEMO Network use more simple hosts such as amoebae, or drosophila flies. In this picture an amoeba (white) eating up a yeast cell (red) is shown.
Figures
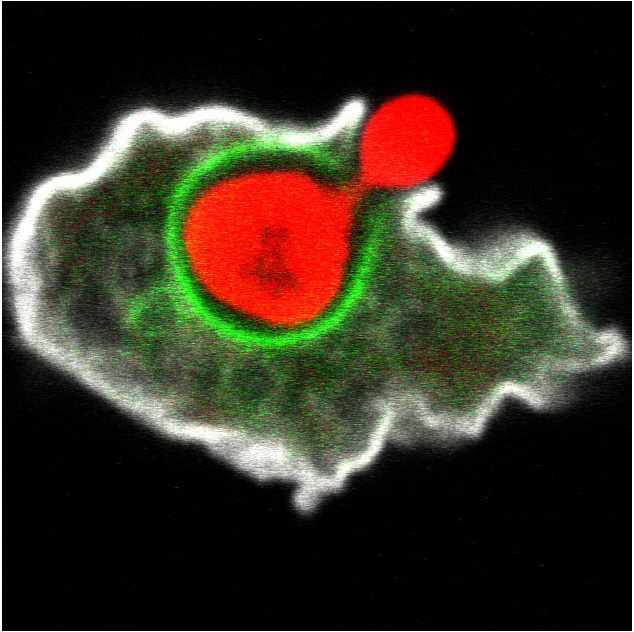
Figure 1: To study infectious diseases, it is not always necessary to infect animals. Researchers in the NEMO Network use more simple hosts such as amoebae, or drosophila flies. In this picture an amoeba (white) eating up a yeast cell (red).
| Last modified 2018/10/12 |