 |
de | fr | en print view![]()
Annual report 2008
PDF versionSummary of the Year’s Activities
The Foundation’s website
Detailed information about all the Foundation’s activities can be found on its website at www.forschung3r.ch. We are happy to report that a large number of people visit the website.
18 projects subsidised
A total amount of Fr. 553,359.85 was paid out for 16 ongoing projects and 2 that were completed during 2008.
Six new projects
Six new projects were approved in 2008 for which a total of Fr. 690,500 was earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Evaluation of lipid fractions for the substitution of serum in cell culture media (109/08) Prof. Paul Honegger and Dr. Marie-Gabrielle Zurich, University of Lausanne. Replacing fetal calf serum by specific additives in culture media for tissue and cell cultures would result in two important improvements, namely no fetuses would be required to obtain the serum and the results from the tissue and cell cultures could be more easily replicated. Lipid fractions would be a possible replacement. Cell cultures are to be used for determining which fraction(s) are most suitable for replacing the serum.
Development of an in-vitro assay for the screening of antischistosomal drugs (110/08) Prof. Jennifer Keiser, Swiss Institute of Tropical Medicine, University of Basle. Schistosomiasis is caused by various trematodes that are harmful to humans. Substances for treating this disease are normally sought using juvenile or adult Schistosomae obtained from infected mice or hamsters. The aim of this project is to determine whether it is possible to screen substances using Schistosomulae isolated from the initial host (snails), thus eliminating the need for testing in Schistosomae and the deliberate infection of animals to obtain them.
Establishment of an organ ex-vivo slice model for cardiovascular research in particular for therapeutic atherosclerosis targeting (111/08) Prof. Patrick Hunziker and Dr. Rahel Bänziger Keel, University Hospital, Basle. Many laboratory animals are required for research into atherosclerosis (causes of the disease, possible treatments, nanomedicine). Prof. Hunziker’s research team at the University Hospital in Basle isolate arteries from mice (that lack the ApoE gene) and human material. The arteries are then further examined in a culture medium. In this way, it is possible not only to reduce the number of laboratory animals required but also to identify any differences in the pattern of development of the disease in mice and humans.
A novel in vitro model for the holistic assessment and optimisation of engineered tissue for functional cartilage repair (112/08) Dr. Zhijie Luo and Prof. Jennifer Kirkham, Leeds Dental Institute, University of Leeds (UK). The aims of the project are, on the one hand, to examine engineered cartilage from the point of view of function and characteristics, and on the other to determine whether the cartilage tissue binds well with the existing, healthy cartilage in the joint. Such testing is often carried out using laboratory animals. Through the cell culture methods developed in this project it should be possible to carry out such tests largely in vitro. A further aim is to develop these alternative methods for use in routine testing.
Generic in-vitro evaluation assay for immunological correlates of protection to replace animal challenge infections (113/08) Dr. Kenneth McCullough and Dr. Artur Summerfield, Institute of Virology and Immunoprophylaxis (IVI), Mittelhäusern. A broad variation in antigens has developed in relation to foot and mouth disease (FMD). In order to ensure that vaccination is successful, the vaccine has to be tailored to the current virus sub-type and tested in animals (challenge infection experiments). The aim of this project is to develop a new, reliable and rapid in-vitro test to replace the use of laboratory animals. It will be carried out as part of an EU consortium that has access to sera from vaccinated animals, reagents and mAbs. This also means that the results of the in-vitro tests can obtain international validation without implicating additional animals.
Reduction in the number of fish used in the fish acute toxicity test (OECD protocol no. 203) (114/08). Dr. Hans Rufli, ecotoxsolutions, Basle. Fish acute toxicity testing often includes extremely high and extremely low doses of the substance in question. By analysing historical data concerning hundreds of agricultural and industrial chemicals, establishing retrospective statistics and simulating earlier testing procedures, it should be possible to reduce the range of doses tested while at the same time obtaining an equally valid evaluation of the toxicity of potential environmentally harmful products. The end result would be a reduction of between 10 and 30% in the number of fish required for acute toxicity testing.
Two projects successfully completed
In vitro replica of the inner surface of the lungs to study particle-cell interaction (89/03) Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne. In this project methods were developed for culturing tracheal epithelial explants and macrophages from pig lung. It was thus possible to determine in vitro the harmful or therapeutic effects of nanoparticles in the lung. Consequently, inhalation studies in laboratory animals (mostly rodents) become superfluous, or at least the number of such tests could be radically reduced. In order to characterise the effects of aerosols, morphological and physiological changes in the cells, including cilium activity, cytokine release and the extent of cell necrosis (LDH release), were measured. These experiments are being continued as part of the international POLYOSA project.
Development of an in-vitro system for modelling bioaccumulation of neutral, ionizable, and metabolically active organic pollutants in fish (100/06) Dr. Beate Escher, EAWAG, Dübendorf. The potential bioconcentration of chemicals in fish is determined in order to estimate environmental risks (OECD test no. 305). This involves the use of a large number of fish. The PAMPA (parallel artificial membrane permeability assay) in-vitro system was further developed as part of this project. Through the use of a specific synthetic membrane (polydimethylsiloxan), a refined stirring technique (provision of oxygen) and appropriate calculation methods it was possible to simulate the diffusion conditions in fish gills. The permeability of 14 reference substances correlated well with the rate of elimination measured in living fish. Exceptions were substances that are metabolised in fish.
3R-Info-Bulletins
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
Host pathogen interactions can be studied in amoebae instead of animals (no. 36, January 2008) Prof. Pierre Cosson and his research team at the University of Geneva have succeeded in determining the virulence of bacteria in single-cell amoebae (Dictyostelium). They observed a similar level of virulence of selected bacteria in amoebae and rodents. Experiments to examine the virulence of bacteria in mice and rats are extremely stressful for the animals. Bulletin no. 36 includes a summary of how many such experiments can be carried out just as effectively in amoebae (Dictyostelium) as well as flies (Drosophila melanogaster) or worms (Caenorhabitis elegans). The amoeba system is simple to use and can be adapted for the special requirements of bacteria (e.g. also fish pathogens).
Bioconcentration of chemicals in fish can be determined in vitro (no. 37, June 2008) Dr. Beate Escher and her research group at EAWAG (Swiss Federal Institute of Aquatic Science and Technology) used the in-vitro PAMPA (parallel artificial membrane permeability assay) system and developed it to the point that diffusion conditions largely corresponded to the situation in fish gills. The use of this new testing procedure means that far fewer fish are required today for evaluating the environmental risk posed by chemicals (OECD test no. 305).
Development of an in-vitro system using lung cells to determine the harmful effects of particles and gaseous substances (no. 38, October 2008). Prof. Marianne Geiser Kamber and her team at the University of Berne have isolated cells from the lungs of pigs sent to slaughter and developed organ-typical cultures, where conditions in the lung could be closely replicated. It was possible to retain not only epithelial cells but also macrophages and even the epithelial secretion, the latter two being important for the removal of particles from the lungs. The use of this test should make it possible not only to replace tests that require many animals but also to obtain information about the processes involved in the potentially harmful impact of a given substance.
Experts Meeting as part of the EU START-UP Project in Basle
On behalf of the 3R Research Foundation, Prof. Peter Maier organised the second Experts Meeting as part of the EU START-UP project (Scientific and technological issues in 3R-alternatives research in the process of drug development and union politics) initiated by ecopa, which was held at the Novartis campus in Basle on 5 September 2008. Invitations were sent to researchers who are familiar with the issue “disease models in laboratory animals” through their daily work. Prof. Maier chaired the meeting, which was attended by 30 experts from Europe and the USA, from seven pharmaceutical companies, who produced proposals for ways of further developing and implementing the 3R principles in disease models involving laboratory animals. The aim of the proposals is to help define future focal points in 3R-relevant EU projects. An analysis of this highly successful meeting is currently being drawn up and will be the subject of a report to be distributed by ecopa to all the participants.
1. Origin of the Foundation
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (association of pharmaceutical companies that carry out research, comprising at present Actelion Ltd, Merck Serono Ltd, Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, and the associated members Bayer (Switzerland) Ltd, Cilag Ltd and Vifor Ltd) and the Foundation for Animalfree Research (animal protection). It was entered in the commercial register on 18th August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
2. Purpose of the Foundation
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods which avoid the use of animals, through grants for research projects. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer practical improvements vis-à-vis standard animal experimentation in line with the 3R motto Reduce, Refine, Replace.
A broad range of projects is sponsored on the condition that they are likely to replace animal experimentation, to reduce the number of animals used or the stress and/or pain suffered. Projects considered must be based on the Foundation’s three principles and are mainly in the bio-medical multidisciplinary field.
3. Organisation of the Foundation
The Administrative Board
The Administrative Board of the Foundation is made up of nine members, three representing the Parliamentary Group for Animal Experimentation Questions (1 seat vacant), two representing animal protection, two from Interpharma and two from the Federal Veterinary Office. Current members are:
Christine Egerszegi-Obrist, member of the National Council, Mellingen (Chairwoman)
Dr. Peter Bossard, Horw (Vice-Chairman)
Chantal Galladé, member of the National Council, Winterthur
Dr. Franz P. Gruber, Doerenkamp-Zbinden Foundation, Küsnacht
Prof. Paul Herrling, Head of Research, Novartis International, Basle
Silvia Matile-Steiner, lawyer, F. Hoffmann-La Roche Ltd., Basle
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld
Dr. Hans Wyss, Director of the Federal Veterinary Office, Berne-Liebefeld
The Evaluation Committee
Prof. Peter Maier, Uster (Chair)
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Prof. Kurt Bürki, Institute of Laboratory Animal Science, University of Zurich
Prof. Clemens A. Dahinden, Institute of Immunology and Allergology, University Hospital, Berne
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne
Prof. Andrew Hemphill, Institute of Parasitology, University of Berne (as from 5.5.2008)
Dr. Kurt Lingenhöhl, Novartis Pharma AG, Basle
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld
Susanne Scheiwiller, B.Sc., Animalfree Research, Zurich (until 19.09.2008)
Dr. Stefanie Schindler, Animalfree Research, Zurich (as from 11.12.2008)
Scientific adviser
Prof. Peter Maier, Uster
Secretary
Ernst P. Diener, lawyer, Münsingen
Auditors
KPMG AG, Gümligen-Berne
Supervisory body
Federal Department of Home Affairs
Articles and statutes of the Foundation
- Deed of foundation dated 13th February, 1987
- Regulations dated 15 May, 1987/11 December, 2008
- Guidelines for awarding research grants dated 15 May, 1987/11 December, 2008
4. Activities during 2008
In its twenty-second year of existence the Administrative Board met twice, namely in May and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2007, the Board addressed the following issues.
Research funds for 2008 were allotted to 16 projects already underway. In addition, 6 new projects were approved, while 22 applications were rejected. The Board also took note of the final assessment by the Evaluation Committee of 2 projects which had been completed in the previous years. Moreover, guidelines for the awarding of research grants concerning the submission of applications were set out and the areas of research were redefined. Finally, the rules and regulations concerning appeals against decisions were revised on the basis of experience gained. In view of the fact that the Foundation’s secretariat has moved to new premises, the agreement concerning how it is run was rewritten. A working group was set up to examine the Foundation’s operational strategy.
At the meeting in May, apart from the financial statements for 2007 and the approval of new projects as well as completed projects, discussions covered strategic questions concerning the improvement of the Foundation’s networking, and an internal working group was set up.
At the December Board meeting, Dr. Peter Bossard was elected to fill the position of Vice-Chairman of the Board for the remaining period of office. Apart from the approval of new projects, discussions also focused in particular on changes in the guidelines for awarding research grants and the appeal process. The Board took note of the Scientific Adviser’s report on his representing the organisation at various events during 2008 and thanked him warmly. Moreover, Prof. Maier reported on the successful experts Meeting in Basle as part of the ecopa Project START-UP (Scientific and technological issues in 3Rs-alternatives research in the process of drug development and union politics). Finally, the internal working group presented its intermediate report on how the 3R principles can be further broadcast. The Board decided to follow the recommendation to follow successfully completed 3R projects more closely to help ensure that newly devised methods are put into practice. Encouragement should be given for suitable methods to be validated. In addition, the Board mandated the working group to continue examining the question of whether and how the Foundation can commit itself as the central office for promoting the 3R principles.
With the support of the scientific adviser, the Evaluation Committee held two meetings during the year, where in particular they assessed new applications and evaluated completed projects. The voluntary work of the members of the Evaluation Committee in this connection is much appreciated.
The Scientific Adviser's tasks included publishing the 3R-Info-Bulletin (as a brochure and on the Foundation’s website at www.forschung3r.ch), writing brief scientific reports in English which present the projects receiving funding, regularly updating the Foundation’s website and managing the “3R Training Course” on the internet. He was also kept busy – as always – advising applicants and project managers, obtaining intermediate reports, evaluating project outlines, dealing with enquiries and explaining why projects had been rejected. Finally, he represented the Foundation at several scientific meetings in Switzerland and abroad, namely as a member of the board of the European Consensus Platform for 3R Alternatives to Animal Experimentation (http://www.ecopa.eu) in Brussels. The Scientific Adviser also put a lot of work into helping to organise the Experts Meeting in Basle under the auspices of ecopa’s START-UP project.
5. Projects subsidised
Overview of the number of applications and approvals
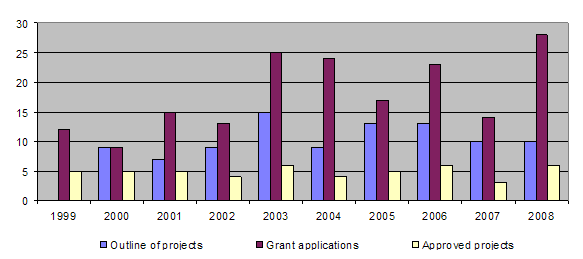
During the year 2 projects were completed (89/03, 100/06). Together with those projects completed earlier, this brings the total of finished projects to 91 out of 114.
The bar-chart shows a reversal in the downward trend in the number of applications received, while the proportion approved varies only slightly. The long-term approval rate for applications is around 30%, giving an average of approximately 5 projects approved each year.
3R Training Course
The Foundation has set up the 3R Training Course internet learning programme to offer individual, specialised further training for people who carry out or supervise animal experiments. This course is available in German and English at http://3R-training.tierversuch.ch. Texts, images, links and documents provide visitors to the site with information on alternatives to animal experimentation. This course has been officially recognised as a further training course under the terms of the Federal Veterinary Office’s Ordinance of 12 October 1998 on the basic and further training of persons involved in animal experimentation (SR 455.171.2). Over the past year, 23 certificates were issued to people who passed the on-line examination.
6. Personnel
Dr. Peter Bossard was elected as Vice-Chairman of the Board for the remaining part of the period of office 2007/2010. Otherwise there were no changes in the Administrative Board. No replacement has yet been found for Dr. Hugo Wick from among parliamentarians. Having taken up a new position, Mrs Susanne Scheiwiller resigned from the Evaluation Committee. New additions to the Committee are Prof. Andrew Hemphill from the Institute for Parasitology at the University of Berne and Dr. Stefanie Schindler from Animalfree Research in Zurich.
7. List of new projects approved in 2008
| 114/08 | Dr. Hans Rufli ecotoxsolutions, Basle Reduction in the number of fish used in the acute fish toxicity test |
| 113/08 | Dr. Kenneth McCullough Institute of Virology and Immunoprophylaxis (IVI), Mittelhäusern Generic in vitro evaluation assay for immunological correlates of protection, to replace animal challenge infection |
| 112/08 | Dr. Zhijie Luo and Prof. Jennifer Kirkham Leeds Dental Institute, University of Leeds, UK A novel in vitro model for holistic assessment and optimisation of engineered tissue for functional cartilage repair |
| 111/08 | Prof. Patrick Hunziker University Hospital, Basle Establishment of an organ ex-vivo tissue slice model for cardiovascular research in particular for therapeutic atherosclerosis targeting |
| 110/08 | Prof. Jennifer Keiser Swiss Institute of Tropic Medicine, University of Basle Development of an in vitro assay for the screening of antischistosomal drugs |
| 109/08 | Prof. Paul Honegger and Dr. Marie-Gabrielle Zurich University of Lausanne Evaluation of lipid fractions for the substitution of serum in cell culture media |
A complete list of projects with summaries of each can be found on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
The brief scientific project reports in English, which are updated once a year, also appear on the website and indicate that almost all projects have progressed well. The project managers’ reports tend increasingly to include helpful images. These reports published on the internet are much appreciated by those involved in the research projects as a platform for presenting their work. From the opposite point of view, this system also enables other researchers all over the world to discover new 3R methods without delay.
8. 3R-Info-Bulletin
In 2008 three more new 3R-Info-Bulletins (ISSN 1421-6590) were published in English and distributed to some 1,000 interested parties. The information bulletins are also published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html), as well as in pdf format.
The latest 3R-Info bulletins are:
List of the other 3R-INFO BULLETINS
9. Financial business
A total of some Fr. 555,300 was paid out for research in 2008 (Fr. 553,360 for grants to research projects and Fr. 1,899 for participation in conferences). Expenditure on current projects (Fr. 553,360) was some Fr. 73,000 under budget (Fr. 626,300); this was principally due to the fact that approximately Fr. 75,000 was paid out for two new projects, while some Fr. 109,000 earmarked for 4 projects was not used because the amounts budgeted for were not required in full. Of the 5% reserve (budgeted at Fr. 51,600) Fr. 17,500 was paid out upon the completion of 2 projects.
Operational expenditure for 2008 amounted to Fr. 228,536.60 (project monitoring and information Fr. 116,800, administrative costs including office infrastructure Fr. 111,730). The total exceeded the budget of Fr. 216,200 by around Fr. 12,300 (5.7%). Administrative costs (Fr. 111,730) were approximately 10% over budget (Fr. 100,000), which can be largely explained by the rise in payment for secretarial services and the cost of increased deliberation concerning strategy issues, changes in the rules and regulations, the high number of applications received (28), the reorganisation of the database and website management. Total expenditure therefore amounted to around Fr. 783,800.
The foundation did not incur any expenditure in connection with the organisation of the Experts Meeting in Basle under the auspices of the ecopa START-UP project since the contribution from ecopa covered all outgoings.
On the income side, the equal financial commitment of the federal authorities and Interpharma represented the basic funding for the Foundation’s activities. In 2008 the federal authorities and Interpharma each granted the Foundation Fr. 425,000. At the end of the year the Federal Veterinary Office promised an additional contribution of Fr. 24,000, which was received at the beginning of 2009. Funds not required immediately were invested in several time deposits of up to 12 months. This produced interest on capital of Fr. 10,300. In addition, income from the 3R Training Course provided Fr. 2,300 and Interpharma’s contribution towards the cost of organising the Experts Meeting in Basle accounted for a further Fr. 8,000.
Total income was therefore around Fr. 870,700 while total expenditure amounted to Fr. 783,800, giving an excess of income over expenditure of around Fr. 86,900. The unused contributions item therefore rose from approximately Fr. 472,300 at the end of 2007 to Fr. 559,200 at the end of 2008.
At the end of 2008 the total earmarked for projects approved by the Board but not yet paid out amounted to Fr. 1,087,295.70. This future liability is covered by Interpharma’s new promise of funding. The Foundation’s credit with this institution amounted to Fr. 2,316,000 at the end of 2008.
The budget for 2009 includes around Fr. 633,000 for current projects and a maximum amount of Fr. 500,000 for new projects.
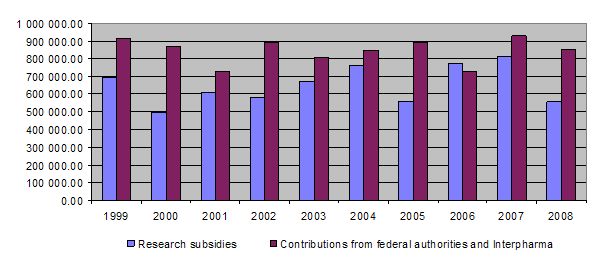
Overview of grants awarded between 1987 and 2008
At the end of 2008 a total of Fr. 15,852,536.30 had been granted for projects and other subsidies, of which Fr. 14,765,240.59 has been paid out so far. Together the federal authorities and Interpharma have contributed Fr. 17,668,000 to the Foundation since 1987.
10-year overview
10. Financial statements
| Profit and loss account 2008 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 425 000.00 | ||
| Contribution from Interpharma | 425 000.00 | ||
| Total contributions | 850 000.00 | ||
| Interest on bank account | 10 300.03 | ||
| Research funds repaid | 0.00 | ||
| Other income | 10 460.99 | ||
| Total income | 870 761.02 | ||
Expenditure | |||
| Research grants | 555 258.84 | ||
| Project supervision and information | 116 806.60 | ||
| Administrative expenses | 111 730.00 | ||
| Total expenditure | 793 795.44 | ||
| Excess income over expenditure | 86 965.58 | ||
| 870 761.02 | |||
| Balance as per 31st December 2008 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 577 443.28 | ||
| Accounts payable | 2 311.16 | ||
| Accounting apportionment assets | 10 598.58 | ||
Liabilities | |||
| Accounting apportionment liabilities | 30 102.20 | ||
| Unused project funds | |||
| Carried forward 1. 1. 2008 | 472 285.24 | ||
| Excess income over expend | 86 965.58 | 559 250.82 | |
| 590 353.02 | |||
| Capital of the Foundation | 1 000.00 | ||
| 590 353.02 | 590 353.02 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 1 087 295.71. | ||
Münsingen, 28 April 2009
3R RESEARCH FOUNDATION
Chairwoman: signed C. Egerszegi
Secretary: signed E. Diener
11. Auditors' report to the Administrative Board
As 3R Research Foundation’s auditors, KPMG AG in Gümligen-Berne has examined the books and the annual financial statements on the basis of current financial reporting standards and recommends that they be approved.
| Last modified 2014/06/04 |