 |
de | fr | en print view![]()
3R-Project 93-04
Development of a novel multicellular 3-dimensional blood brain barrier in vitro model
Omolara Ogunshola
Institute of Veterinary Physiology, Vetsuisse Faculty University of Zürich, Winterthurerstr. 260, 8057 Zürich, Switzerland
larao@access.uzh.ch
Keywords: rat; astrocytes; brain; epithelia; endothelia; epithelia; cns, brain disorders; pharmacology; barrier systems in vitro; cell cultures: 3d cultures; cell cultures: co-cultures; cell cultures: organ-specific; cell cultures: primary; cell cultures: reconstituted tissue; reduction; replacement
Duration: 3 years Project Completion: 2010
Background and Aim
The blood-brain barrier (BBB) plays a crucial role in preserving physiological brain homeostasis. Notably disruption or dysfunction of the BBB constitutes a well-described hallmark of many socially and economically important pathological states, thus understanding regulation of BBB is crucial to reduce/prevent injury/disease progression. Although different animal models are used to study various diseases characterised by loss of BBB integrity the cellular and molecular mechanisms involved are still only poorly understood. Since high complexity of the brain makes interpretation of in vivo data challenging BBB studies are frequently performed using highly simplified in vitro models. Despite this the translation of in vitro data to the in vivo situation remains inadequate. Remarkably, many models fail to address important features such as the 3D structure of blood vessels, the complex cellular interactions that occur in vivo or include the 3 specific cell types that comprise the BBB.
The aim of this project was to establish a cell culture system that contains the three major cell types that form the BBB in vivo : endothelial cells, astrocytes and pericytes. We have developed, characterised and validated an innovative in vitro system that is an accurate representation of the BBB in vivo and additionally allows dynamic movement and reorganization of cells in response to a changing environment - significantly refining current in vitro models. This model can potentially reduce and replace animal experimentation with in vitro testing and aid identification and refinement of new therapeutic targets and protective strategies. New innovations such as this therefore have potentially wide applications in basic science as well as medical and pharmaceutical industries.
Method and Results
The BBB cultures consist of endothelial cell, astrocytes and pericytes mixed in a ratio of 1:5:1 and suspended in a collagen matrix that solidifies at room temperature and is overlaid with media. The matrix allows cellular movement and enables each cell to display its specific unique morphology as occurs in vivo. During early development (up to 4 days) the culture manifests many endothelial structures such as cysts, intercystal connections and tube-like structures that appear analogous to those observed during vascular development in vertebrates such as blood islands, primary vascular plexus and angiogenic blood vessels (Fig 1). After 5 days our in vitro culture has stabilised and closely mimics the structure and interactions of cells comprising the BBB in vivo (see Figure 2). Validation of our model shows it correlates well to the in vivo situation in many important aspects. The blood vessels produced are hollow demonstrating the presence of a patent lumen. Furthermore astrocyte and/or pericyte interactions are required for adequate induction of well-established barrier properties such as proper localised adherens and tight junction complex formation as well as efficient vascular polarity. Challenging the cultures with stressors, such as exposure to hypoxia or mannitol that induce changes in barrier characteristics, convincingly reproduces the expected alterations in specific cellular interactions as well as protein localisation and expression. Thus the model is dynamic and accurately responsive to environmental influences.
We have used our model to differentiate the distinct roles of both astrocytes and pericytes at the BBB. Overall, our data suggest that during development pericytes play an important pro-angiogenic role whereas astrocytes are anti-angiogenic accelerating tube stabilization and maturation. In contrast during adverse conditions such as hypoxia or drug exposure our findings indicate that both astrocytes and pericytes significantly contribute to vascular stability, although to different degrees, possibly through synergistic signalling. We are now using our model to investigate the action of a number of compounds at the BBB. Furthermore we have performed cDNA array analysis at different stages of development to identify genes instrumental in induction, maintenance and break down of the BBB. A first manuscript with detailed description of the characterisation and validation of the model is currently being peer reviewed.
This versatile system will undoubtedly improve our understanding of the importance of cell-cell interactions and the mechanisms underlying BBB regulation during both physiological and pathological situations. We hope the future will see a wide use of this and related models in the BBB field.
Conclusions and Relevance for 3R
This system provides a unique opportunity to more easily study the BBB and reduces the need for difficult, invasive animal experiments. As such it has wide applications in basic research as well as medical and pharmaceutical industries. Furthermore, this model system will not only provide information on specific cellular interactions and signals that promote induction of BBB formation during development, but is readily manipulated and subjected to different insults to aid understanding of BBB breakdown. Thus utilisation of this model means research can be more focused and directed to the specific roles of individual cell types and barrier function as a whole with minimal use of animal experimentation.
In conclusion this work promotes a means for refinement of potential therapeutic tools and strategies prior to animal testing, and ultimately reduces animal experimentation, consumables and personnel costs.
(see also 3R-INFO-BULLETIN Nr. 42)
References
1. Zlokovic, B. V., The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57 (2), 178 (2008).
2. Pardridge, W. M., Blood-brain barrier biology and methodology. J Neurovirol 5 (6), 556 (1999).
3. Al Ahmad, A., Gassmann, M., and Ogunshola, O. O. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol 218 (3), 612 (2009).
4. Kaur, C. and Ling, E. A., Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res 5 (1), 71 (2008).
5. Al Ahmad, A., Gassmann M., and Ogunshola, O. O. Astrocytes and pericytes differentially modulate BBB characteristics during development and hypoxic insult. JCBFM (2010), in print.
Figures
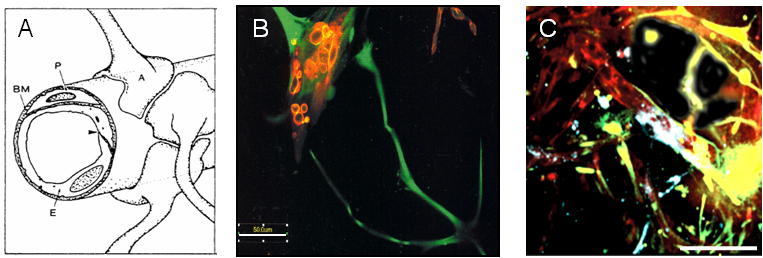
Figure 1: Brightfield and fluorescent images showing development of model over time reveals many endothelial structures (cysts, intercystal connections and vessel-like structures) analogous to those observed in vivo (eg blood islands, primary vascular plexus and angiogenic vessels).
Figure 2
Diagrammatic representation of the BBB in vivo (A, from Abbot 1989). Cellular interactions in our in vitro model are analogous to the organisation in vivo (B and C). Red: endothelial cells; green/yellow: astrocytes; blue: pericytes.
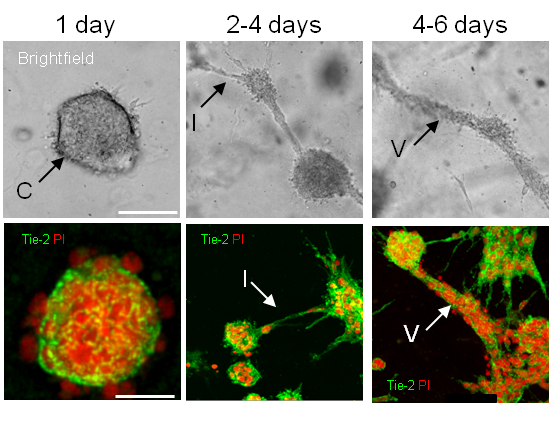
Figure 2
| Last modified 2018/10/12 |