 |
de | fr | en print view![]()
3R-Project 43-95
Short term assay for liver cell activated neurotoxic drugs
Peter Maier1and Arend Bruinink2
Institute of Toxicology, ETH Zürich, 8603 Schwerzenbach, Switzerland
1present address: University of Zürich, 8057 Zürich, Switzerland2present address: EMPA, 9014 St. Gallen, Switzerland.
peter.maier@uzh.ch, arie.bruinink@empa.ch
Keywords: chicken; rat; brain; liver; toxicology; cell cultures: co-cultures; cell cultures: organ-specific; cell cultures: primary; reduction; replacement; toxicity testing: xenobiotics
Duration: 3 years Project Completion: 1999
Background and Aim
The identification of chemicals with a potential neurotoxic activity is an important aspect in toxicity testing of drugs, agricultural chemicals and industrial products. Current risk assessment guidelines recommend testing organophosphorus compounds (OP) in vivo in hens, since this species is highly sensitive. The relatively high costs, low "throughput" and massive animal distress involved in testing compounds in whole animal experiments led to the development of in vitro tests making use of human and animal neuroblastoma cell lines. However, a severe limitation of these tests is that they are unable to detect compounds requiring metabolic activation in order to exert their neurotoxicity as is the case for most OPs. The aim of this project was to establish a cell culture model capable of performing this metabolic activation, with the view of establishing it as an in vitro screening system to detect both specific and non-specific neurotoxic compounds.
Method and Results
Primary rat hepatocytes were used as a metabolic activation system (1). After 3 days in culture (2), the hepatocytes were exposed to the test compound. Six hours later, cultured chicken embryonic brain cells (3), a sensitive target for neurotoxic chemicals, were exposed to the supernatant of the treated hepatocytes. Stable metabolites released from intact liver cells (4) and exerting their toxicity in the cocultured brain cells might also have the potential to reach the nervous tissue in an intact organism.
Cylophosphamide (CP: 10 - 300 µM, activated mainly by CYP 2B1/2) was used as a positive control to characterise the metabolic competence of the coculture system. Isofenphos (IF: 10 -100 Bayer AG) was used as a representative organophosphate with a high selective neurotoxicity in animals and humans. Brain cells directly exposed to the two chemicals showed no signs of toxicity. When exposed to the supernatant collected from hepatocytes treated with the parent compounds, a concentration-dependent toxicity was detectable, indicating that the chemicals were converted to stable toxic metabolites by the hepatocytes. A similar dose-dependent toxicity of hepatocyte derived metabolites detectable was found with IF.
However, bioactivated CP metabolites induced both cytotoxicity (MTT-assay) and a parallel inhibition of acetylcholinesterase (AchE) activity in a dose-dependent fashion, whereas IF was able to inhibit AchE activity even at nontoxic concentrations (5). This corresponds well to the specific neurotoxic activity of this compound in vivo and demonstrates that the inhibition of an organ-specific function (in this case function of the cholinergic nerves) can occur independently of general cytotoxicity.
(see also 3R-Info-Bulletin 13)
updated version 2007 (pdf file)
Conclusions and Relevance for 3R
The established sequential use of hepatocytes (activation system) and chicken brain cells (target) is suitable for the detection of the activation of OP and other xenobiotics (e.g. CP). The cytotoxicity obtained with the supernatant from hepatocytes treated with the parent compounds indicates that stable metabolites are formed; in vivo these would have a high probability of reaching the brain. Furthermore, this experimental model can discriminate between the induction of nonspecific cytotoxicity (CP) and specific neurotoxic effects (inhibition of AChE activity without cytotoxicity as shown with IF). Accordingly, chemicals with a cholinotoxic activity can be identified, making further testing in animal models superfluous. Used as a primary screening procedure, this test has the potential of reducing the number of animals needed to assess the toxicity profile of a compound.
Clearly the sequential and the co-culture approaches do not provide a complete answer as to the on-going processes in an intact organism. In the case of organophosphates, it is already known that this group of chemicals is potentially neurotoxic. In the case of pharmaceuticals, it may be important to know whether the metabolites reach brain cells or whether their access is inhibited by the well established blood-brain barrier (BBB) and choroid plexus (CP). This question can be addressed using in vitro cell culture systems in which the specific transporters and activities of the BBB (e.g. Cecchelli et al.2007) or the CP (Baehr, Reichel et al. 2006) are expressed. Either the original compound or its metabolites can be tested and new information can be obtained about molecular structures which allow or prevent access to the brain.
(see also 3R-INFO-BULLETIN Nr. 13)
Published updated Version 13/2007 (pdf)
References
1. Maier P., Saad B. and Schawalder H.P. (1994) Effect of periportal- and centrilobular-equivalent oxygen tension on liver specific functions in long-term rat hepatocyte cultures, Toxic. in Vitro 8, 423-435.
2. Bruinink A., Rasonyi T. and Sidler C. (1998) Differences in neurotoxic effects of ochratoxin A, ochracin and ochratoxin-alpha in vitro. Natural Toxins 6: 173-177.
3. Milosevic N., Schawalder H.P. and Maier P. (1999) Kupffer cells mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes, Europ. J. Pharmacol. 368, 75-87.
4. Maier P., Milosevic N. Bruinink A, (2000) Co-cultures: mimicry of cellulkar interactions within a tissue and between organs - consequences for toxicity testing. In: Progress in the Reduction, Refinement and Replacement of Animal Experimentation (Eds. M. Balls, A.M. van Zeller and M.E. Haller), pp. 249-256, Elsevier, Development in Animal and Veterinary Sciences, 31, The Netherlands.
5. Brunink A., Yu D. and Maier P. (2002) Short term assay for the identification of neurotoxic compounds and their liver derived stable metabolites, Tox. In Vitro 16: 717-724.
6. Cecchelli, R., Berezowski, V., Lundquist S. et al. (2007). Modelling of the blood-brain barrier in drug discovery and development. Nature Reviews, 6 (in press).
7. Baehr, C., V. Reichel, et al. (2006). "Choroid plexus epithelial monolayers - a cell culture model from porcine brain." Cerebrospinal Fluid Res. 3: 13-24.
8. Bruinink, A. (2008). In vitro toxicokinetics and dynamics: modelling and interpretation of toxicity data. Preclinical Development Handbook. S. C. Gad. New York, John Wiley & Sons, Inc.: in press.
Figures
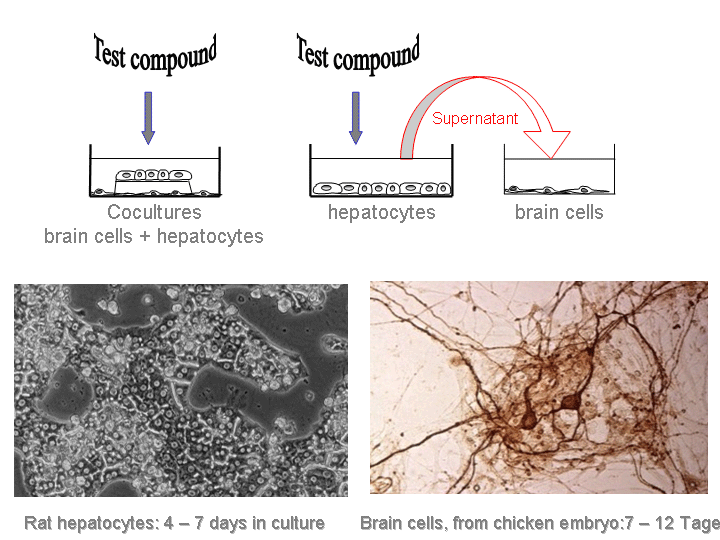
Figure 1: The sequential type of exposure mimics more closely the in vivo situation. The organ-specific (tissue-specific) toxicity in liver and brain cells as well as the toxicity of the parent test compound and its metabolites can easily be dissected in separate hepatocyte or brain cultures.
| Last modified 2018/10/12 |