
3R-INFO-BULLETIN 51
| | November 2013Authors
Professor Artur Summerfield is Chair of Veterinary Immunology. His research interests focus on understanding protective innate and adaptive immune responses to foot-and-mouth disease (FMD). The aim is to provide new tools to control the disease, which causes extremely painful lesions in cloven-hoofed animals such as ruminants and pigs. The basic research that is performed in this area should result in an improvement in currently-available vaccines and furnish tools for the optimal selection of vaccines. FMD was the subject of Nils Lannes` doctoral thesis. He is currently working as a postdoctoral scientist at the University of Fribourg, in Switzerland. Address:
Artur Summerfield
artur.summerfield@ivi.admin.ch Institute of Virology and Immunology (IVI)
CH-3147 Mittelhäusern, Switzerland
Editor
Ernst B. Hunziker, Scientific Adviser of the 3R Research Foundation |
Generic in-vitro assays for immunological correlates of protection against foot-and-mouth disease to replace animal challenge infections
The Institute of Virology and Immunology (IVI) is the Research Unit of the Federal Veterinary Office. It is dedicated to the study of animal health, particularly to the diagnosis, surveillance and control of highly-infectious epizootic diseases. And in collaboration with the Vetsuisse Faculty of the University of Bern, it assumes a teaching role, as well as it undertakes research tasks, in the fields of virology and immunology, with a focus on livestock and zoonotic viral diseases. Although Switzerland and Europe are currently (December 2013) free of FMD, globally, this highly contagious viral disease is the cause of severe economical losses. For this reason, all work pertaining to the FMD-virus is conducted in the high-containment facility of the IVI in Mittelhäusern.
Vaccine matching of foot-and-mouth disease vaccines
Vaccination against foot-and-mouth disease (FMD) represents an essential element in controlling and combating outbreaks, which can otherwise have disastrous consequences such as during the FMD outbreak in the UK in 2001. This is pertinent to regions in large parts of the developing world in which the FMD virus (FMDV) is endemic, as well as during an epidemic in FMDV-free areas such as Europe. Nevertheless, successful vaccination against FMDV requires selection of the appropriately matching vaccine strain providing protection against a particular circulating field virus. This problem originates from the existence of seven known serotypes of FMDV, with which a high antigenic variation of the virus is observed. In addition, subtypical antigenic variation within a serotype is under constant evolutionary change due to the high mutation rate of FMDV. For these reasons, continuous vaccine testing and modification in the light of recent antigenic changes to the virus is required. Traditionally, vaccines are tested and selected using vaccination-challenge experiments in cattle. Such procedures are not only extremely expensive, but are also environmentally and ethically problematic, considering the severe animal suffering associated with disease development, and the requirement that all animals be slaughtered at the end of the experimentation.

Animals culled during the FMDV outbreak in the UK in 2001
Substituting vaccine potency tests by assays enabling a correlation of in-vitro tests based on immunological principles of antibody-based effector immune responses operating in vivo
In the frame of the EU FP7 project FMD-DISCONVAC (KBBE-2008-1-3-02), serological correlates of vaccine-induced protection were systemically evaluated by several members of the consortium (http://fmddisconvac.net). These included classical serum neutralization tests, liquid phase blocking ELISAs as well as tests based on measuring opsonizing antibodies. The latter approach was funded by the 3R-Project 113-08 with the aim to obtain robust in-vitro alternatives to such challenge-infection tests. To this end, the present project integrated with the EU project FMD-DISCONVAC. Although vaccine-induced protection can be predicted when high levels of virus-neutralizing antibodies are induced, this does not apply for vaccinates with relatively low levels of antibodies. So far, no alternative tests applicable to such sera have been developed and systematically applied to vaccine testing. Thus, the approach selected goes beyond virus neutralization tests, and also enable the functional analysis of non-neutralizing antibodies with respect to FMDV destruction and the induction of antiviral activity.
Fcγ receptor-expressing plasmacytoid dendritic cell cultures as sensors of FMDV immune complexes
Various cellular systems were established to test the influence of immune complexes obtained from vaccinated cattle and pigs. These included an assay measuring enhanced infection of bovine monocyte-derived dendritic cells by immune complexed-FMDV and for the porcine system enhanced IFN-alpha responses by plasmacytoid dendritic cells (pDC). Both assays represent a very sensitive method to measure opsonizing antibodies. The titres of these antibodies were similar to neutralizing titres, when antigenically related viruses from the same serotype were employed. However, sera cross-reacted also with non-neutralized isolates of multiple serotypes when tested in this assay. Both uncomplexed virus and immune complexed virus stimulated pDC via Toll-like receptor 7. An additional finding of potential importance for strain-specific differences in virulence and/or immunogenicity was that pDC activation by FMDV strongly differed between viral isolates. Altogether, our results indicate that opsonising antibodies can have a broader reactivity than neutralizing antibodies and may contribute to antiviral responses induced against antigenically distant viruses. This work was published (Lannes et al., 2012 Vet Res 43:64).
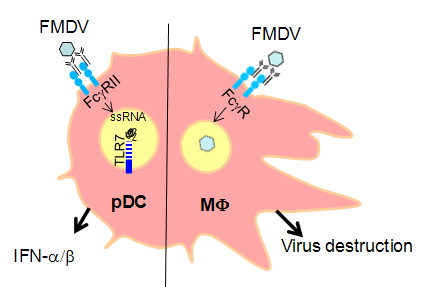
Fig 1
Immunological background: FcR-mediated anti-FMDV responses induced in plasmacytoid dendritic cells (pDC) and macrophages (MΦ) as examples of antibody-mediated effector functions independent of direct virus neutralization.
To systematically investigate the relationship of opsonizing antibodies with protection using a large panel of sera, we also have established a reporter system to measure opsonizing antibodies against FMDV. This is based on bovine FcRII (CD32)-expressing murine RAW 264.7 macrophages. The principle of this test is that the RAW 264.7 cells are resistant to FMDV infection in the absence of antibodies but become infected and die when the virus is complexed with antibodies. Our results demonstrate that this test is highly sensitive, similar to the test using bovine MoDC. We tested a panel of cattle sera from vaccination/challenge experiments to determine whether these novel tests have the ability to measure correlates of protection. In fact, a better correlation to the vaccine dose was observed with opsonizing antibody levels when compared to neutralizing titres. However, the number of tested sera is not yet sufficiently high to conclude on the relationship to protection.
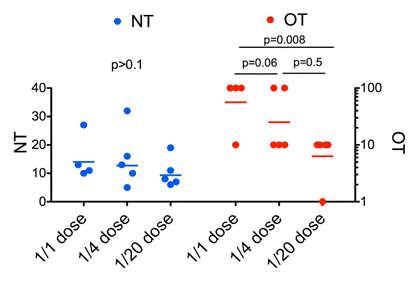
Fig 2
Relationship of neutralizing and opsonizing antibodies to vaccine dose and protection. Comparison of neutralizing (NT, blue, left y-axis) and opsonising (OT, red, right y-axis) antibodies induced by vaccination of cattle with the FMDV vaccine strain O3039 reactive with FMDV O/Bulgaria/2011. Three of four animals were protected with the full dose, two of four with the half dose and no protection was obtained with the quarter dose.
Relevance for 3R
This project has demonstrated the immunological relevance of opsonizing antibodies against FMDV, at least in vitro. Such antibodies, even if not neutralizing, are likely to enhance innate resistance against FMDV through induction of IFN-α and phagocyte-mediate virus elimination. Together with other methods developed in parallel by members of the FMD-Disconvac consortium, which include improved liquid-phase blocking ELISA, avidity ELISA and bioinformatic information, as well as 146S quantification in vaccine preparations, we conclude that in general vaccination/challenge experiments to determine vaccine matching and efficacy can in future be replaced by immunization experiments only. This will reduce the suffering of animals, reduce the biosafety risks associated with challenge experiments, and reduce the costs of vaccine testing both for research and routine purposes in the field of FMD.
PDF version of this Bulletin No. 51
References:
- Lannes N., Python S. and Summerfield A. (2012) Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Veterinary Research 2012, 43:64, doi:10.1186/1297-9716-43-64.
- Lannes N, Summerfield A. (2013) Regulation of porcine plasmacytoid dendritic cells by cytokines. PLoS One. 2013;8(4):e60893. doi:10.1371/journal.pone.0060893.




