
3R-INFO-BULLETIN 41
| | October 2009Authors Epithelix was founded in 2006 by 4 scientists (Ludovic Wiszniewski, Samuel Constant, Jean-Paul Derouette, and Song Huang) from Geneva University. One of Epithelix’s missions is to promote and implement 3R principles, which were explicitly expressed in the statutes of the Company. As Chief Scientific Officer, Dr. Song Huang, a cellular and molecular biologist, is responsible for production and research. Mireille Caulfuty, a skillful and competent technician, performed most of the work. Epithelix was founded in 2006 by 4 scientists (Ludovic Wiszniewski, Samuel Constant, Jean-Paul Derouette, and Song Huang) from Geneva University. One of Epithelix’s missions is to promote and implement 3R principles, which were explicitly expressed in the statutes of the Company. As Chief Scientific Officer, Dr. Song Huang, a cellular and molecular biologist, is responsible for production and research. Mireille Caulfuty, a skillful and competent technician, performed most of the work.
Address: Song Huang
song.huang@epithelix.com Mireille Caulfuty
mireille.caulfuty@epithelix.com Epithelix Sàrl
14 Chemin des Aulx
CH-1228 Plan-les-Ouates
Genève, Switzerland www.epithelix.com
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
A novel in-vitro cell model of the human airway epithelium
The present project (No. 106-07) funded by the 3R Research Foundation, contributes to the development of a standard operating protocol (SOP) for toxicity tests using a novel in-vitro cell model of the human airway epithelium. This model, developed and produced by the company Epithelix, is commercially available under the brand MucilAir™. It represents a morphologically and functionally differentiated 3D epithelium which can be maintained at a homeostatic state for more than one year. MucilAir™ has been successfully introduced onto the market and contributes to the reduction of animal experiments[*].
Relevant and organ-related targets
In a number of in-vitro and ex-vivo models, the differentiated organ- or tissue-specific structures and functions can be maintained (2). This preferentially in freshly isolated primary cell cultures, precision lung or brain slices. However, in most of them the in-vivo like situations can be maintained only a few hours or days, which makes it difficult to use them for repeated, chronic or long-term toxicity tests. Requirements for an ideal cell culture model includes structures and functions typical for a defined target-organ or tissue affected after human exposure e.g. the human respiratory tracts to volatile chemical compounds.
Tissue-specific structures and functions
The present novel in-vitro cell model of the human airway epithelium has been developed by the company Epithelix (3) and commercialized under the brand name MucilAir™. The 3D cultures are prepared from epithelial cells isolated and purified after enzymatic digestion of pieces of nasal/bronchial biopsies (collected in the hospitals, with the consent of the donors). The primary cells are cultured at an air-liquid interface under serum free conditions and in a homeostatic state for more than a year. Furthermore, the cultures are adapted to high throughput screens (HTS).
The epithelium (MucilAir™) expresses organ-specific structures (cilia) and functions, including defense mechanisms (mucus production and cilia beating) (4). Similar to the in-vivo airway epithelia, mucus is synthesized, which serves to trap the inhaled particles. With the help of cilia beating, harmful particles can often be removed. The loss or reduction of cilia beating can be used as an indicator of toxicity together with non-destructive endpoints allowing chronic and long-term toxicity testing.
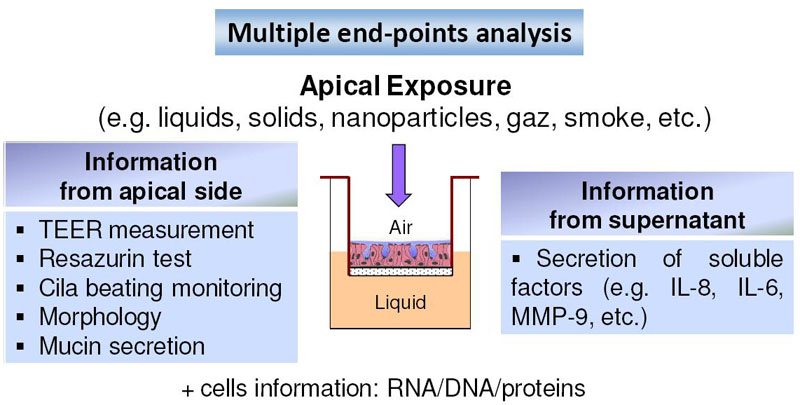
Fig. 1: Strategy of toxicity testing
The chemical products, in the form of liquid, solid or gas, are applied on the apical surface (exposed to the air). Following a standardized protocol, non-destructive endpoints are determined allowing chronic and long term toxicity testing.
Water can be toxic
The epithelial cells, especially the airway epithelial cells, have the ability to synthesize and release inflammatory mediators, such as IL-8, in case of virus and bacterial infections. These inflammatory mediators would recruit the leukocytes to fight the virus and bacteria. Applying physiological saline solution (0.9% NaCl) on the apical surface of the epithelium (Figure 1) resulted in a dramatic increase of IL-8 release (Figure 2). This demonstrates an intact defense pathway typical for human airway epithelia. The absence of air is sensed as a danger by the airway epithelia cells, defending themselves by the release of an inflammatory mediator such as IL-8.
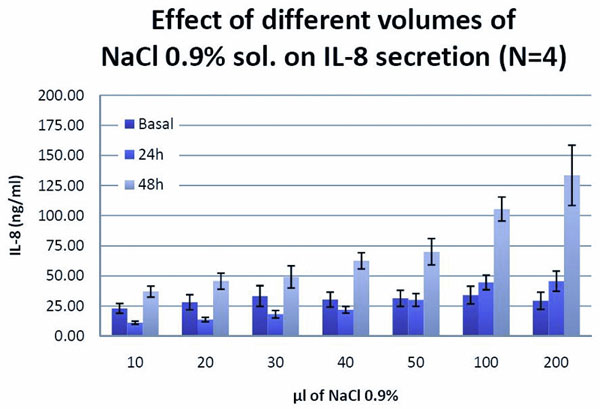
Fig. 2: The effect of physiological saline solution
Applying physiological solution (0.9%, NaCl) on the apical surface of MucilAir™, an inflammatory response, characterized
Beyond the in-vitro model
Toxicity tests in general are designed to minimize variance, bias, and the potential for false-positive and false-negative results. The same is true for in-vitro cell models. With the help of the 3R grant, the potential of MucilAir™ was evaluated as a tool for assessing the toxic effects of chemicals. A number of tasks have been resolved: a standard protocol for acute toxicity testing and several endpoints has been established with 9 chemicals (publication in preparation). The results demonstrated that the reproducibility is excellent; there was almost no batch-to-batch variation. In addition, partnerships have been established with industrial partners for validating MucilAir™.
Standardized protocols
The standardized protocol can be summarized as following: the chemical products, in the form of liquid, solid or gas, are applied on the apical surface (exposed to the air, Figure 1). We have chosen a one-hour incubation time in a volume of 200 μl in 24 well plates. 24 hours after the exposure, several non-destructive endpoints were evaluated, such as TEER measurement, cilia beating, cell viability test (Resazurin assay), measurement of released cell mediators (IL-6, IL-8, MMP-9). Representative EC50 viability for nicotine is given in Figure 3. The data obtained suggest that MucilAir™ is not only a reliable and reproducible in-vitro cell model for assessing the toxicity of the chemical in epithelial tissue but is also suitable for inhalation toxicity tests, such as in the area of occupational health problems.
A washing step (three times within one hour) is carried out before the test chemical is added to get rid of accumulated mucus which allows exposure levels at the cells to be defined more precisely. The selection of non-destructive endpoints, such as TEER, Resazurin assay, allows chronic and long term toxicity testing. Depending on the purpose of the test, additional (destructive) endpoints such as RNAs, DNAs and proteins extractions for genomics and proteomics analysis can be included.
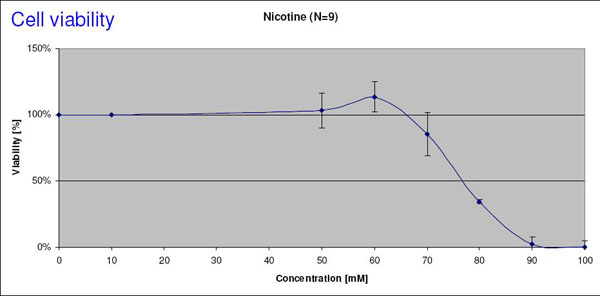
Fig. 3a
Effect of Nicotine on Cell viability
Dose-response curve of cell viability (Resazurin Test) in MucilAirTM after exposure to Nicotine. The EC50 value is estimated to be 78 mM.
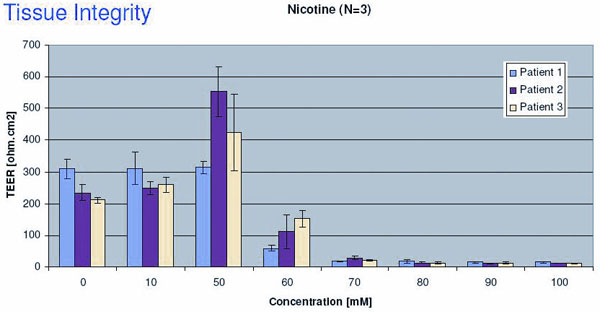
Fig. 3b
Nicotine induced changes in Tissue integrity
EC50 value of Nicotine determined by TEER measurements is about 55 mM. TEER is a very sensitive endpoint and it is also very easy to perform. The tests have been done on three different batches of MucilAir™, in triplicate. Judging by the standard derivation, the variation is small, and the reproducibility is good.
3R benefits
The expertise gained in this project allowed us to provide customers in the chemical as well as the pharmaceutical industry with testing services. Certainly these investigations replaced or prevented a number of investigations in laboratory animals. In the future, we believe that in the area of toxicity pathway related and targeted testing (1), such organ specific, human cells based system like MucilAir™ with intact structure and function will play a prominent role. Our final goal is to have MucilAir™ validated by competent authorities such as ECVAM, OECD or ICH in order to be used in regulatory toxicity testing.
PDF version of this Bulletin No. 41
References:
- US National Research Council (NRC) 2007: Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington DC.
- Hartung, T. and Daston, G (2009) Are in-vitro tests suitable for regulatory use? Toxicological Sciences 111: 233-237.
- www.epithelix.com
- Berube, K., Aufderheide, D., Breheny, R., Clothier, R., Combes R., Duffin, R., Forbes, B., Gaça, M., Gray, A., Hall, I., Kelly, M., Lethem, M., Liebsch, M., Merolla, L., Morin, JP., Seagrave, J., Swarts, MA., Tetley TD. And Umachandran, M. (2009): In-vitro models of inhalation toxicity and disease – The report of a FRAME workshop. ATLA 37, 89-141.
| [*] | Need for reliable in-vitro cell modelsThe number of animals used for toxicity testing of chemicals is increasing each year. The advent of the European Legislation on chemicals (REACH) will greatly accentuate this trend: it is estimated that between 30 – 60 000 chemicals will need to be tested within the next 10 years. For this, depending on the tests required and the number of chemicals registered, 10-50 million animals have to be sacrificed. The total cost for testing these chemicals amounts to 1.3 – 9.5 billion Euros and this mainly due to expensive animal tests (e.g. reproduction toxicology). Any new alternative methods allowing high throughput screening, targeted testing of a relevant toxicity pathway in differentiated human cells might contribute to reduce in-vivo testing, as outlined in the 21st century approach (1). One possibility of alternative testing is the use of in-vitro cell models representing specific target cells in our body such as fibroblasts, epithelial cells, endothelial cells, muscle cells, neuronal or astroglial cells. Cells can be maintained as monolayers, suspensions, cocultures and in 3- dimensional arrangements (3D cultures). Up to now, the majority of in-vitro cell models are not of human origin or they are based on immortalized cell lines which, by gaining the capacity to proliferate indefinitely, lose their ability to differentiate. As a consequence, they fail to express physiological characteristics of the corresponding tissues in vivo. Often they show impaired inter- and intra-cellular signaling, loss and gain of chromosomes, accumulation of mutations, shortage of oxygen supply, lack of biotransformation capacities or lack of defense mechanisms. Such alterations would impair testing based on toxicity pathway and on specific relevant targets. The present project is a contribution toward a cell models based on human cells and expressing a stable in-vivo like pattern of structures and function typical for a tissue in the nasal/bronchial airways which is often exposed to toxic insults. |
 Epithelix was founded in 2006 by 4 scientists (Ludovic Wiszniewski, Samuel Constant, Jean-Paul Derouette, and Song Huang) from Geneva University. One of Epithelix’s missions is to promote and implement 3R principles, which were explicitly expressed in the statutes of the Company. As Chief Scientific Officer, Dr. Song Huang, a cellular and molecular biologist, is responsible for production and research. Mireille Caulfuty, a skillful and competent technician, performed most of the work.
Epithelix was founded in 2006 by 4 scientists (Ludovic Wiszniewski, Samuel Constant, Jean-Paul Derouette, and Song Huang) from Geneva University. One of Epithelix’s missions is to promote and implement 3R principles, which were explicitly expressed in the statutes of the Company. As Chief Scientific Officer, Dr. Song Huang, a cellular and molecular biologist, is responsible for production and research. Mireille Caulfuty, a skillful and competent technician, performed most of the work.



