
Figure 1

Author: Martin Clauss1 and Andrej Trampuz2
11Kantonsspital Liestal, Department of Orthopedics, 4410 Liestal, Switzerland
2CHUV, Infectious disease service, 1001 Lausanne, Switzerland
E-Mail: martin.clauss@ksli.ch, andrej.trampuz@chuv.ch
This in-vitro approach allows the quantification of biofilm formation on implants, human bone grafts and bone-graft substitutes by means of microcalorimetry. The method has the advantage of allowing the detection of bacterial biomass without removing the biofilm from the surface of the specimen.
Keywords: bacteria; bacteria; orthopedics; infectiosity; microcalorimetry; replacement; infectiosity; toxicity testing: biomaterials / implants
Method
Infection of the surgical site after bone transplantation cannot be prevented completely. The majority (70-90%) (1) of infections associated with orthopaedic foreign bodies is caused by staphylococci which typically grow on the surface in a specialized structure known as biofilm (2). Conventionally, the infectious properties are tested in animal studies. The present in-vitro approach allows to quantify biofilm formation on implants, human bone grafts and bone-graft substitutes by means of microcalorimetry (3,4). Heat measured derives from metabolic processes in living cells (bacteria) mainly due to daughter cell formations after cell division. The method has the advantage of allowing the detection of bacterial biomass without removing the biofilm from the surface (5) of the specimen.
We use a 48-channel batch calorimeter (thermal activity monitor, model 3102 TAM III; TA Instruments, New Castle, DE, USA, Fig 1). Temperature differences between the sample (Ts) and a thermally inert reference (Tr) are continuously measured in a heat sink (Ths) at variable temperatures controlled to within 0.0001°C at a sensitivity of 0.25 µW (Fig 2). Samples containing biofilm are prepared in vitro and transferred to the microcalorimeter for analysis. After thermal equilibration (15 mins), samples are lowered into the measurement position.
3R effect
In-vitro biofilm analysis on irregular surfaces of medical devices will help to screen various materials prior to in-vivo testing and thus reduce the number of animals needed. The microcalorimeter allows parallelscreening of up to 48 samples (Figure 1) with a high reproducibility, precision and limited costs per sample, making it an ideal instrument for in-vitro biofilm screening.
References/ Links
1. Gristina AG. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987;237-4822:1588.
2. Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs 2005;28-11:1062-8.
3. Lerchner J, Wolf A, Buchholz F, Mertens F, Neu TR, Harms H, Maskow T. Miniaturized calorimetry - a new method for real-time biofilm activity analysis. J Microbiol Methods 2008;74-2-3:74-81.
4. Buchholz F, Harms H, Maskow T. Biofilm research using calorimetry - a marriage made in heaven? Biotechnology Journal 2010;5-12:1339-50.
5. Clauss M, Trampuz A, Borens O, Bohner M, Ilchmann T. Biofilm formation on bone grafts and bone graft substitutes: comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater 2010;6-9:3791-7.
Figures
Figure 1
48-channel batch calorimeter
Figure 2
Schematic drawing of a single microcalorimetric unit.

Figure 1
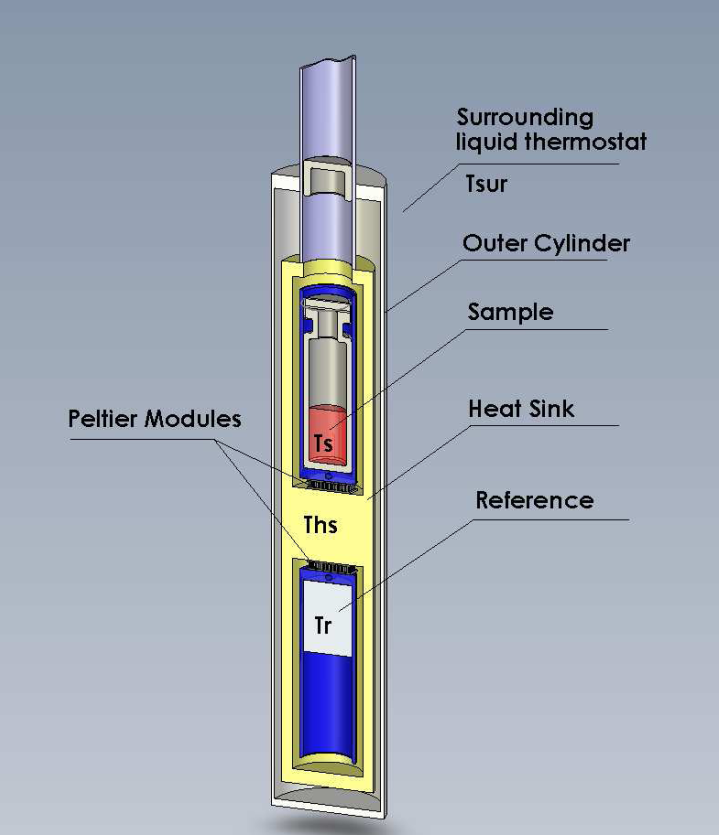
Figure 2