
3R-INFO-BULLETIN 8 - August 1996
| | The AuthorHans-Peter Hauri is professor of cell biology at the Biozentrum of the University of Basel.
A trained zoologist, he has worked in various research fields. Most recently he has focused on protein traffic and organelle biogenesis in the secretory pathway of mammalian cells.  Prof. Hans-Peter Hauri Prof. Hans-Peter Hauri
Abteilung Pharmakologie
Biozentrum, Universität Basel
Klingelbergstr. 70
CH-4056 Basel
Tel. +41 61 267 2222
Fax +41 61 267 2208
E-mail: Hauri@ubaclu.unibas.ch |
Regulation of Digestion in Cell Culture
In a project supported by the Foundation Research 3R, an in vitro model using human intestine epithelial cells has been successfully established
Enterocytes, enzymes and digestion
Epithelial cells, termed enterocytes, line the lumen of the small intestine and are responsible for the terminal digestion of carbohydrates and proteins. This task is accomplished by a set of digestive enzymes associated with the luminal brush border membrane of the enterocytes. How are these enzymes regulated?
Despite considerable effort by many laboratories it has not been possible to establish a non-transformed permanent enterocyte cell line in a differentiation-competent state that would allow the study of digestion at a cellular and molecular level in cell culture. Studies on the regulation of digestive enzymes in vivo are met with limitations because (a) the developmental expression of these enzymes is different in humans and laboratory animals, (b) individual enzymes vary along the crypt-villus axis and the proximal-distal direction, (c) it is impossible to differentiate between intrinsic mechanisms of the enterocytes and those that depend on epithelial-mesenchymal interactions.
The Caco-2 cell culture model
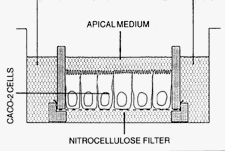
The human adenocarcinoma cell line Caco-2 has been introduced as a model system to study various aspects of intestinal biology (ref.1). Caco-2 cells form a monolayer of polarized cells when grown on a permeable support in a two-chamber system and after confluency spontaneously develop a number of features characteristic for differentiated enterocytes including the expression of brush border enzymes such as lactase (cleaves milk sugar), sucrase-isomaltase (cleaves cane sugar and breakdown products of starch and glycogen), and dipeptidylpeptidase IV (ref.2).
 Cac0-2 monolayer in the transmission electron microscope
Cac0-2 monolayer in the transmission electron microscope
 Cac0-2 cells: a) Localization of dipeptidylpeptidase IV in the brush border by immunofluorescence microscopy using a specific monoclonal antibody
Cac0-2 cells: a) Localization of dipeptidylpeptidase IV in the brush border by immunofluorescence microscopy using a specific monoclonal antibody
 Same cells visualized by phase contrast microscopy
Same cells visualized by phase contrast microscopy
Previous studies have shown that Caco-2 is a valuable model to elucidate mechanisms underlying membrane traffic and polarity in epithelial cells (refs. 2,3), but can this model also be used to study the regulation of digestive enzymes?
Regulation of digestive enzymes in Caco-2 cells
We tested, with particular emphasis on lactase, how these enzymes are regulated by hormones and activators of second messenger systems (ref. 4). Activators of the cAMP pathway, including vasoactive intestinal peptide, were found to rapidly stimulate lactase synthesis and to inhibit sucrase-isomaltase synthesis while dipeptidyleptidase IV was unaffected. A similar but considerably slower response was noted with hydrocortisone. An activator of the diacylglycerol pathway inhibited sucrase-isomaltase synthesis but had no effect on lactase and dipeptidylpeptidase IV. Thyroxin selectively stimulated dipeptidyleptidase IV synthesis. Thus the three enzymes reacted in a characteristic way to the different stimuli, whereby lactase and sucrase-isomaltase showed opposite behavior.
Possible relevance for human physiology
Caco-2 cells offer an interesting possibility of studying the regulation of brush border enzymes at the molecular level. These experiments set the stage for a more detailed analysis of the modulating factors including substrates for the individual digestive enzymes. To what extent the present findings obtained in a transformed cell line are relevant for the human small intestine in vivo is presently unknown. Similar experiments with intestinal explants that can be maintained in organ culture for up to two days may give a partial answer. Nevertheless, it is worth mentioning that during human development, the levels of circulating vasoactive intestinal peptide parallels the levels of lactase in the intestine. This suggests that cAMP-mediated lactase induction seen in Caco-2 cells is likely to be of physiological relevance.
Understanding the regulation of lactase is of nutritional importance. About 10% of Swiss and over 50% of the entire human population cannot tolerate milk after they reach adulthood due to a gradual drop in the expression of this enzyme. These individuals suffer from osmotic diarrhea after the ingestion of milk and therefore are unable to profit from its nutritional advantages.
References
1. Zweibaum, A., Laburthe, M., Grasset, E. and Louvard, D. (1991). The use of cultured cell lines in studies of intestinal cell differentiation and function. In: Handbook of Physiology (Field, A. and Frizzel, R.A., eds.) Vol 4, pp. 223-255. Amer. Physiol. Soc. Bethesda.
2. Louvard, D., Kedinger, M., and Hauri, H.-P. (1992). The differentiating intestinal epithelial cell: Establishment and maintenance of functions through interactions between cellular structures. Ann. Rev. Cell Biol. 8, 157-195.
3. Matter, K., Brauchbar, M., Bucher, K. and Hauri, H.-P. (1990). Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell 60, 429-437.
4. Hauri, H.-P., Sander, B. and Naim, H. (1994). Induction of lactase biosynthesis in the human intestinal epithelial cell line Caco-2. Eur. J. Biochem. 219, 539-546.
Prof. Hans-Peter Hauri
