
3R-INFO-BULLETIN 15
| | September 2000The AuthorsProf. Dr. Paul Honegger1 is head of a research group at the Institute of Physiology, University of Lausanne. Together with Dr. Beatriz Pardo-Merino2 and other coworkers, he developed the present 3D-cell culture model. current address:
1Institut de Physiologie Université de Lausanne,
Ecole de Médicine,
CH 1005 Lausanne,Switzerland.
Email: paul.honegger@iphysiol.unil.ch
2Centro de Biología Molecular Severo Ochoa, Laboratorio 450,
Campus de la Universidad Autónoma de Madrid,
Cantoblanco,
28049 Madrid, España
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Aggregating brain cell cultures: Investigation of stroke related brain damage
The present project, supported by the 3R Research Foundation, provides a cell culture model, which is particularly useful for mechanistic studies on processes involved in ischaemia. Brain cells are prepared from embryonic rat or mouse brain tissue and cultured as 3-dimensional (aggregating) cell cultures. The cell culture model is complementary to investigations with animal models and will contribute to a reduction of in vivo studies.
The brain is highly vulnerable to ischaemia
The interruption of blood supply to the brain (ischaemia) deprives brain cells of glucose and oxygen, causing irreversible brain damage within minutes.[*] The brain is particularly vulnerable to ischaemia because of 1) the very high rate of oxidative metabolism in this organ, requiring a continuous supply of oxygen and glucose, 2) the metabolic interdependence of neurons and astrocytes, two types of brain cells, and 3) the sensitivity of neurons to disruption of ion homeostasis brought on by ischaemia.
Cells in ischaemic brain tissue undergo a number of changes: they rapidly lose their energy stores, their membranes become depolarised, calcium loads increase, reactive oxygen species are produced and excitotoxic effects are found. These biochemical changes are followed by irreversible changes to cellular structures and cell death (necrosis, apoptosis). The neurons are particularly sensitive to injury, whereas reactive glial cells (astrocytes and microglia) tend to amplify and propagate the tissue damage.
Animal models versus cell cultures
Today, research on ischaemia relies mainly on animal models. Unfortunately, such studies involve a high de-gree of discomfort for the animal. In vivo approaches involve procedures such as the surgical occlusion of major arteries, or (less invasive) the induction of reproducible infarcts in a selected area of the brain by means of artificially induced thrombosis. The animal models appear to closely reproduce the characteristics of gray matter ischaemic injury in humans.
Various types of cell culture models have been developed to study mechanistic aspects of cerebral ischaemia. Each type of model has specific advantages and disadvantages (Table 1). However, neither animal models nor culture systems are able to reproduce all the patterns of ischaemic pathologies encountered in humans.
Table 1 Comparison of in vitro Culture Models
| In Vitro Model | Specific advantages | Disadvantages |
|---|
| Monolayers | | tumor cells | - defined cell population | - high variability | | embryonic brain cells | - directly observable in microscope
- electrophysiological intact
- easy access for biochemical (secretion, staining) analysis | - partial loss of brain specific functions
- ischaemia inducible only under harsh conditions
- limited cell-cell interactions | | Cell Aggregates | | 3D-cultures with embryonic brain cells | - high yield and reproducibility
- undergo extensive maturation, interactions which affect metabolic changes and the cascade of ischaemia induced pathogenesis | - limited access for microscopic observations and electrophysiological cell-cell analysis | | Tissue Slices | | from adult brain | - preserve to some extent the organotypic structure of the brain regions | - low yield and low reproducibility
- repetitive sampling not possible |
|
Ischaemic pathways can be induced
Aggregating brain cell cultures (also called brain spheroids) are prepared from embryonic rat or mouse brain tissue [1]. The cells can be maintained for several weeks as floating, suspended cultures under continuous gyratory agitation (swirling at 80 rpm) in an incubator (Fig. 1).
Ischaemic conditions can be induced in the aggregating cell cultures simply by stopping the gyratory agitation for different lengths of time, and then restoring the cells to normal culture conditions [2]. The effects of transiently stopping the circulation of medium and oxygenation to the cells are assayed one week after the insult. Using cell-type specific biochemical markers of normal brain cell function (for example, glutamic acid decarboxylase (GAD) enzyme activity), it was found that the transient immobilisation caused selective neuronal cell death and delayed glial reactions similar to those found in incomplete ischaemia in vivo [3]. Similar neurotoxicity was observed in cultures subjected to hypoxia or hypoglycemia [4].
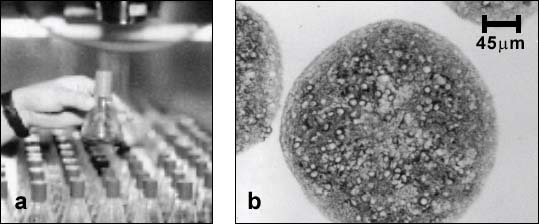
Figure 1:
a) Each flask contains over a thousand of spherical cell aggregates. b) Sections of differentiated aggregates showing stained neurons (cell bodies and neurites stained for microtubule associated protein (MAP-2)) surrounded by unstained glial cells.
...and prevented
Glutamate stimulation and excessive calcium influx are thought to be critical events in ischaemia-induced neurotoxicity. Accordingly, it might be possible to protect neurons by inhibiting these steps along the ischaemic cascade. Our results with aggregating brain cell cultures indicate that this is indeed the case: Nifedipine, a blocker of voltage-gated calcium channels, and MK801, an antagonist of the NMDA ionotropic glutamate receptor, were both able to inhibit the loss of GAD activity (Fig. 2).
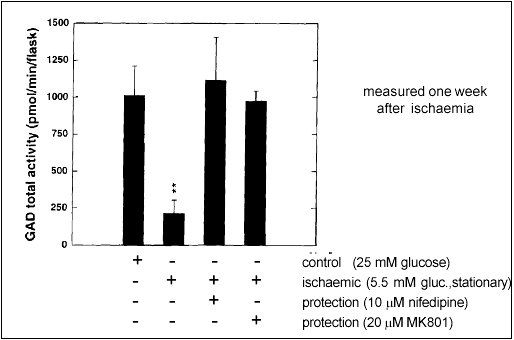
Figure 2: Protection from ischaemia induced neurotoxicity by a calcium channel blocker (Nifedipine) or an NMDA receptor antagonist (MK801). GAD activity is used as a marker of normal brain cell function.
Aggregating brain cell cultures, a promising in vitro model
In summary, aggregating brain cells exhibit unique features useful for the study of pathogenic mechanisms involved in cerebral ischaemia. The culture system permits easy handling, repetitive sampling and post-injury follow-up studies. Data obtained so far demonstrate that many of the fundamental pathogenic processes involved in ischaemia can be studied and recognised in aggregating cell cultures. A thorough knowledge of the mechanisms involved in ischaemic cell damage will allow us to precisely identify the critical steps and cellular and subcellular targets in this process. This in turn will enable the development and testing of potential therapeutic agents designed to inhibit or counteract specific steps in the ischaemic cascade of neurodegeneration.
Published updated version of this Bulletin 15/2007 (PDF)
References:
- Honegger, P., and B. Pardo. Separate neuronal and glial Na+,K+-ATPase isoforms regulate glucose utilization in response to membrane depolarization and elevated extracellular potassium. J. Cereb. Blood Flow Metab. 19: 1051-1059, 1999.
- Pardo, B., and P. Honegger. Aggregating brain cell cultures as a model to study ischemia-induced neurodegeneration. Toxicology in Vitro 13: 543-547, 1999.
- Pardo, B., and P. Honegger. Selective neurodegeneration induced in rotation-mediated aggregate cell cultures by a transient switch to stationary culture conditions: a potential model to study ischemia-related pathogenic mechanisms. Brain Res. 818: 84-95, 1999.
- Pardo B., and P. Honegger. Evaluation of aggregating brain cell cultures as a model for studying ischaemia-related neurodegenerative processes. In: M. Balls, A.-M. van Zeller and M.E. Halder, eds., Progres in the Reduction, Refinement and Replacement of Animal Experimentation. Elsevier Sci., 2000, pp. 241-247.
| [*] | The risk of a cerebrovascular accident such as a stroke doubles every 10 years from the age of 45 onwards. At the age of 75, the incidence reaches about 2%. Ischaemia-related brain damage is thus one of the most important health problems in industrialised countries. This situation is likely to become further aggravated as the average age of the population continues to rise.
The extent of brain tissue damage depends on the brain region affected as well as on the duration and degree of decreased tissue blood suply. In regions of incomplete ischaemia, selective neuronal damage and delayed cell death are observed. A promising therapeutic strategy would be to develop medications which can interrupt early stages in the neurode-generative cascade. To find such medications, the cellular and molecular reactions involved in pathogenicity need to be fully elucidated. Accordingly, research in this area has recently gained high priority, in both areas of basic research and drug development. |




