Figure 1

Author: Barbara Rothen-Rutishauser, Institute of Anatomy, University of Bern, Baltzerstrasse 2, CH-3000 Bern 9, Switzerland
E-Mail: barbara.rothen@ana.unibe.ch
Particle-lung cell interactions can be investigated in a triple cell culture in vitro model of the human airway wall. It is possible to analyze the cellular interplay and response of epithelial cells, human blood monocyte derived macrophages and dendritic cells after exposure to particles.
Keywords: human; dendritic cells; epithelia; lung; macrophages; lung diseases; cell cultures: organ-specific; reduction; replacement; toxicity testing: nano- ultrafine particles
Method
In this 3D model of the human epithelial airway barrier model, monolayers of two different human epithelial cell lines, alveolar type II cells (A549) (Rothen-Rutishauser et al., 2005) and bronchial cells (16HBE14o-) (Blank et al., 2007) were grown on a microporous membrane in a two-chamber system. After isolation and differentiation of human blood derived monocytes into macrophages and dendritic cells, these cells were added at the apical side and at the basal side of the epithelium respectively. Cell densities of macrophages and dendritic cells within the culture were quantified using the specific surface markers CD14 and CD86 (Rothen-Rutishauser et al. 2008 and 2010). Epithelial integrity, cell morphology and cell differentiation were analyzed after exposure to nanoparticles (Müller et al. 2010) and compared with in vivo data. Furthermore an air-liquid exposure system has been evaluated and tested (Lenz et al. 2010) and the effects of nanoparticles in epithelial mono-cultures as well as the triple cell co-culture have been determined (Brandenberger et al., 2010a). In several publications cellular response, uptake of hybrid nanoparticles and cytotoxicity were investigated (Brandenberger et al. 2010a and b, Clift et al. 2010).
3R effect
Numerous in vivo studies, most of them with rodents, are carried out world-wide in order to investigate pulmonary adverse effects of inhaled volatile chemicals or nanoparticles. Although the complex process of lung cancer, or lung fibrosis including COPD cannot be fully mimicked, it is expected that a substantial amount of investigations can be carried out with the proposed 3D epithelial airway barrier system due to the fact that the interaction of different cell types can be studied and that the cells are of human origin.
References/ Links
1. Rothen-Rutishauser B, Kiama SG, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol.Biol 32(4):281-9 (2005).
http://www.ncbi.nlm.nih.gov/pubmed/15640437
2. Blank F, Rothen-Rutishauser B, Gehr P. Dendritic cells and macrophages form a transepithelial network against foreign particulate antigens. Am J Respir Cell Mol Biol 36:669-677 (2007).
http://www.ncbi.nlm.nih.gov/pubmed/17272826
3. Rothen-Rutishauser B, Blank F, Muehlfeld Ch, Gehr P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Exp Opin Drug Metabol Toxicol (Invited Review) 4(8):1075-1089 (2008).
http://www.ncbi.nlm.nih.gov/pubmed/18680442
4. A recently published book chapter describes in detail the practical aspect of the triple cell co-culture:
Lehmann A, Brandenberger Ch, Blank F, Gehr P, Rothen-Rutishauser B. A 3D model of the human epithelial airway barrier. In: Alternatives to animal testing. Eds: Yarmush ML, Langer RS. Artech House. In press. (2010)
http://www.artechhouse.com/Detail.aspx?strIsbn=978-1-60807-011-4
5. Müller L, Comte P, Czerwinksi J, Kasper M, Mayer ACR, Gehr P, Burtscher H, Morin JP, Konstandopoulos A, Rothen-Rutishauser B. A new exposure system to evaluate the toxicity of (scooter) exhaust emissions in lung cells in vitro. Environ Sci Technol 44(7):2632-38 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/19586954
6. Lehmann AD, Parak WJ, Zhang F, Zulqurnain A, Röcker C, Nienhaus GU, Gehr P, Rothen-Rutishauser B. Fluorescent-magnetic hybrid nanoparticles induce a dose-dependent increase of the pro-inflammatory response in lung cells in vitro correlated with intracellular localization. Small 6(6):753-62 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/20333695
7. Lenz AG, Karg E, Lentner B, Dittrich V, Brandenberger C, Rothen-Rutishauser B, Schulz H, Ferron GA, Schmid O. A dose-controlled system for air-liquid interface cell exposure and its application to zinc oxide nanoparticles. Part Fibre Toxicol 6:32 (2009).
http://www.ncbi.nlm.nih.gov/pubmed/20015351
8. Brandenberger C, Rothen-Rutishauser B, Mühlfeld C, Schmid O, Ferron GA, Maier KL, Gehr P, Lenz AG. Effects and uptake of gold nanoparticles deposited at the air-liquid interface of a human epithelial airway model. Toxicol Appl Pharmacol 242(1):56-65 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/19796648
9. Brandenberger Ch, Mühlfeld Ch, Zulqurnain A, Lenz AG, Schmit O, Parak WJ, Gehr P, Rothen-Rutishauser B. Quantitative evaluation of cellular uptake and trafficking of gold nanoparticles with different surface coatings. Small (2010), in press.
http://www.ncbi.nlm.nih.gov/pubmed/20602428
10. Clift MJ, Gehr P, Rothen-Rutishauser B. Nanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternative. Arch Toxicol (2010), in press.
http://www.ncbi.nlm.nih.gov/pubmed/20499226
Figures
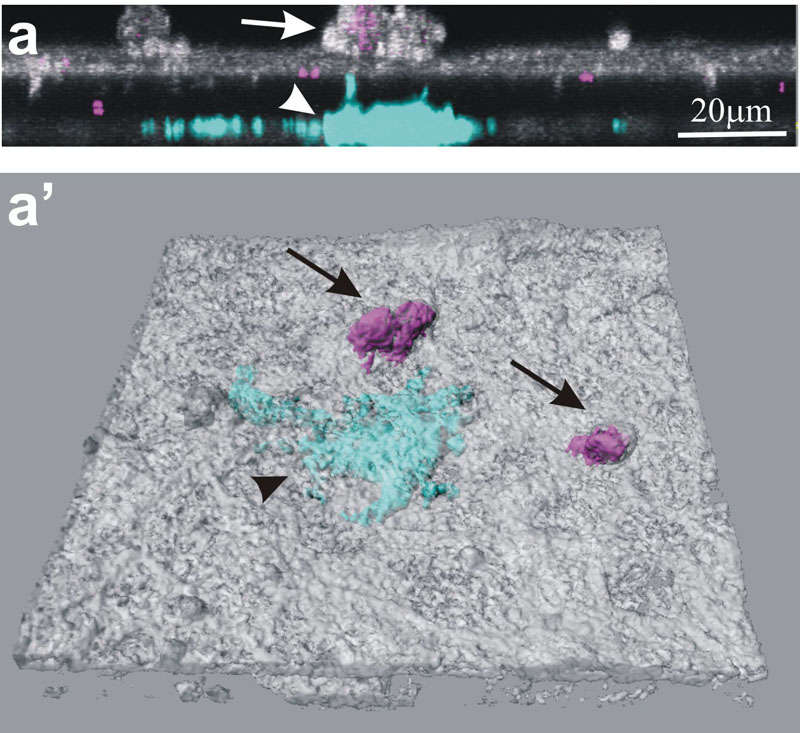
Laser scanning microscopy image of the triple cell co-culture model. Epithelial cells (white), monocyte-derived macrophages on top (purple,
black arrows), and monocyte-derived dendritic cells underneath (light blue, white arrow) the epithelium are shown. The same data set is shown in a) and a’). a) xz-projection, a’) 3 dimensional surface rendering of all cells, the epithelial cells are made transparent (from Lehmann et al., 2010). Reproduced with permission from Artech House.

Figure 1