
3R-INFO-BULLETIN 21
| | September 2002The AuthorThe project was carried out by Maria Ponec, associate professor at the Department of Dermatology, Leiden University Medical Centre. Her research focuses on keratinocyte biology using conventional keratinocyte cultures and human skin equivalents. Current address: Maria Ponec m.h.ponec-waelsch@lumc.nl
Department of Dermatology
Leiden University
Medical Centre, Sylvius Laboratory
Wassenaarseweg 72
2333 AL Leiden
The Netherlands
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Identification of new human skin irritation markers for tests with human skin reconstructs
The use of cultured tissues and cells from humans, the target species of interest, is one of the advantages of in vitro work. The 3R Research Foundation is pleased to present the results of this successful project (Nr. 65-98), in which human skin and reconstructs were used to identify new reliable markers suitable for testing chemicals and consumer products for skin irritation without the need of animal tests.
Mild skin irritants require new markers
Severe acute irritation and skin corrosion manifests itself as acute local cytotoxicity of cutaneous cells. This can be detected in vitro as morphological or functional changes in cultured cells, e.g. by standard tests for viability (damaged mitochondria [MTT test]; membrane damage [NRU test]), release of arachidonic acid metabolites (inflammation markers) or changes in cytokine production.
However, there is an increasing need to screen mild irritants that do not induce acute cytotoxicity and produce little or no morphological changes[*]. This requires that new sensitive markers be found (marker proteins) that accurately represent these subtle functional changes occurring in human skin after the application of mild or low concentrations of irritants.
Proteomics, the method of choice
Any new epidermal protein that appears in human skin tissue after exposure to a known skin irritant might have the potential to be a new marker. How can such a protein be identified? The genome of each cell can code for a vast number of proteins (300 000 - 106). To analyse the expression pattern of the proteins (expression-proteomics), the proteins are separated on the basis of their charge and molecular weight (two-dimensional polyacrylamide gel = 2D-PAGE) and then visualised by markers or stains (spots). Differences between the protein expression patterns from treated and untreated tissue samples are detected by computer assisted analysis (image analysis) of the ratios of protein spot intensities (Fig. 1).
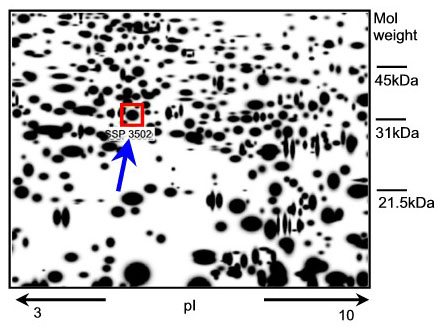
Fig. 1: A representative 2D-PAGE image representing epidermal proteins in excised skin treated with 2% SLS. The SLS-induced epidermal protein (spot SSP3502) is indicated in the box (see arrow). Mass spectrometry used for identification revealed that this spot is HSP27.
Identification of a new marker protein
A well-known skin irritant, sodium lauryl sulphate (SLS), was applied topically to excised human skin. The epidermal proteins whose expression significantly increased or decreased in a dose-dependent fashion were identified. Seven proteins that could serve as potential new epidermal markers for skin irritation were characterized by mass spectrometry (1). Of the seven candidates, the 27 kDa heat shock protein (HSP27) was the protein that reacted with the strongest upregulation following irritation of the skin (Fig. 1).
To determine the value of HSP27 as a putative sensitive marker of irritation, immunohistochemistry was performed on skin specimens exposed to non-toxic SLS concentrations. A strong nuclear staining for HSP27 was observed in SLS-treated excised skin specimens, whereas in the vehicle controls only cytoplasmic staining was observed.
Moreover, nuclear staining was seen after exposure to other irritants, such as nonanoic acid and benzalkonium chloride, and after exposure to UV. In addition, SLS treatment also induced nuclear HSP27 immunoreactivity in vivo after human volunteers were exposed to SLS (Fig. 2).
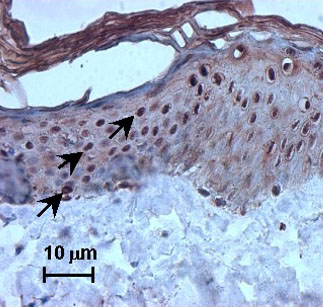
Fig. 2: SLS-induced nuclear translocation of heat shock protein (HSP) 27 (strong nuclear staining, see arrow) on excised human skin topically treated with 2% SLS.
Reconstructed human skin for screening tests
In addition to tests for cutaneous irritancy, there is also an increasing need for rapid and reproducible in vitro methods to assess the effectiveness of topical preparations and their percutaneous absorption rates. Reliable, reproducible screening tests require standardised samples. Freshly excised skin is not widely available in large amounts, nor can it be cultured over extended periods, thus it is unsuitable for extensive and long-term studies on skin irritation. Furthermore, since the time course and degree of a skin irritancy reaction in vivo is strongly related to the barrier capacity of the stratum corneum (SC) (Fig. 3), a suitable in vitro model must adequately mimic the skin barrier function in humans.
Various human skin recombinants have been reconstructed in vitro; many of these mimic the native tissue to a high degree. They are generated by growing differentiated keratinocyte cultures for approx. 2 weeks on cell-free dermal substrates such as deepidermized dermis (DED) derived from cadavers, inert filters (Fig.3), fibroblast-populated collagen matrices or lyophilised collagen-GAG membranes cross-linked by chemical agents. Models on filters are commercially available (e.g. SkinEthicTM and EpiDermTM). Morphological studies have shown that these skin equivalents form a multilayered epithelium (2). Furthermore, they display characteristic epidermal ultrastructures and express markers of epidermal differentiation (Fig. 3).
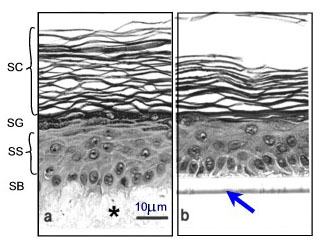
Fig. 3: Morphology of epidermis reconstructed on two different dermal substrates: (a) de-epidermized dermis DED (*) as a cell free natural matrix, (b) inert support (filter see arrow). SB=stratum basale, SS=stratum spinosum, SG=stratum granulosum, SC=stratum corneum
The reconstructs respond somewhat differently
We evaluated whether the expression of HSP27 in the nucleus can also be used as a parameter to evaluate potential skin irritants or cellular stress factors such as UV exposure in the skin reconstructs on DED or EpiDermTM. Following exposure to SLS or UV light, stress-induced nuclear relocalization of HSP27 was observed in both DED and EpiDermTM models. Addition of vitamin C to the reconstructs prevented this reaction, indicating that the presence of vitamin C may control the response to the irritants or stress (3).
With the in vitro approach toward the detection of new pathways
The analysis of epidermal proteins from irritant-treated skin by 2D-PAGE is a promising approach to detect new putative sensitive markers of human skin irritation. The response in reconstructed human skin leads to a more comprehensive understanding of the mechanisms involved (e.g. the role of vitamin C). In the near future this will result in a complete replacement of animal tests for irritant testing.
Whether the new marker can be used also in skin sensitisation testing, is not known. The cellular and molecular mechanisms involved (4) and the conditions for cocultures of skin equivalents with immuno-competent cells (e.g. dendritic cells) still have to be explored.
References:
- Boxman ILA, Hensbergen, PJ, Van Der Schors RC, Bruynzeel DP, Tensen CP, Ponec M: Proteomic analysis of skin irritation reveals the induction of HSP27 by sodium lauryl sulphate in human skin. Br J Dermatol 146:777-785,(2002).
- Ponec M., Weerheim A., Kempenaar J, Mulder A, Gooris GS, Bouwstra J, Mommaas AM: The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol 109:348-355 (1997).
- Boxman ILA, Kempenaar J, de Haas E, Ponec M: Induction of HSP27 nuclear immunoreactivity during stress is modulated by vitamin C. Exp Dermatol (2002) in press.
- Ulrich P: Human monocyte derived dendritic cells as in vitro indicators fort contact allergic potential of chemicals. 3R Research Foundation, Project 67-99.
| [*] | Skin irritation and corrosiveness testingDermatotoxicologists have to protect workers and consumers from chemicals and consumer products that have the potential to i) irritate the skin or the eyes (irritation), ii) elicit toxic responses in combination with UV-light (phototoxicity), iii) corrode the skin (irreversible damage) or iv) sensitise the skin (caused by immunological mechanisms). Traditionally these tests have been carried out in animals. Critics point out that the results have a limited predictive value for humans, and oppose the suffering involved in these in vivo tests (e.g. the Draize test for skin irritation). Recent advances in our understanding of skin biology have resulted in the development of in vitro tests using cell cultures, skin excised from rat, pig or humans, and reconstructed human skin models. International acceptance of animal-free approaches is high for the detection of severe acute irritants, of phototoxic compounds and of corrosives using a sequential testing strategy with a weight-of-the-evidence analysis. However, acceptance is very limited for the identification of mild irritants. The highest predictivity would be obtained in ethical clinical studies with human volunteers, complemented by studies with human skin explants or reconstructed human skin. However suitable in vitro markers to predict and quantify human skin irritation have been lacking up until now. Skin sensitisation cannot be detected ex vivo so far, due to the absence of a sufficient number of living immunocompetent cells (Langerhans cells) in the available human skin reconstructs. |




