 |
de | fr | en Druckansicht ![]()
3R-Project 139-14
An in-vitro microvascular model of the endothelial barrier
Marietta Herrmann1, Laurent Barbe2
1 AO Research Institute Davos, 7270 Davos Platz, Switzerland
2 CSEM, 7302 Landquart, Switzerland
marietta.herrmann@aofoundation.org, laurent.barbe@csem.ch
Keywords: cardiovascular; endothelia; angiogenesis; angiogenesis; cell cultures: intact tissue; tissue engineering
Duration: 2 years Project Completion: 2017
Background and Aim
Pericytes are an important component of the endothelial barrier in capillaries and other microvessels. They are vascular mural cells which reside in the basement membrane together with endothelial cells. Their spatial distribution, abundance and relationship with endothelial cells reflect the function of individual tissues. For example in organs that are characterized by high gaseous and metabolic exchange rates, the distribution of pericytes is such that diffusion is minimally hindered [1]. Interestingly, during the past few years, it has become evident that perivascular cells may also represent a physiological reservoir of adult mesenchymal stem cells (MSCs) [2]. MSCs have the potential to differentiate into adipogenic, osteogenic and chondrogenic lineages, and are therefore widely used for the regenerative engineering of musculoskeletal tissues. However, most of these studies have drawn on MSCs that have been expanded in monolayer cultures, since suitable models and techniques for achieving this in a more physiologically relevant three-dimensional environment are not available. At the same time, the culture of MSCs in monolayers is known to exert a great influence on their phenotypic and functional parameters [3].
The aim of this project is to establish an in-vitro model for the study of perivascular cells in a physiologically relevant context.
Method and Results
The project aims to establish a model system embodying the most important parameters that cells perceive in their physiological environment: cell-cell interactions, the extracellular matrix, paracrine factors and mechanical forces, such as flow and shear, which are particularly important in regulating the phenotype and behaviour of vascular cells. Although microfluidic systems hold the potential to closely mimic the cellular microenvironment at spatial and temporal levels, they are usually comprised of endothelialized microchannels; only a few include also a perivascular compartment. Hence, cell-cell interactions between vascular and perivascular cells have been mostly studied in static three-dimensional co-culturing systems [4]. In a recent study conducted by the group of Abraham Stroock, both approaches were combined for the first time [5; 6]. In this model, perfused microvessels are embedded within a collagenous hydrogel. Endothelial cells are applied to the circulating medium and are able to adhere to and align with the microchannels during the first few days of culturing. Using this system, the microvessels could be successfully cultured for up to 2 weeks, during which time they were remodeled into units with an elliptical cross-section and underwent angiogenesis. In this project, we will employ a similar system, in which a hydrogel will be used to imitate the perivascular compartment containing pericytes and perfused endothelial-cell-lined microchannels (Figure 1).
In the first part of the project, we will focus on the development of the microfluidic chamber. Here, we will test different vessel configurations (Figure 2A, B) and hydrogels, and optimize the rate of perfusion to accord with physiological parameters. Thereafter, cell-seeding and imaging protocols will be established. Fluorescence labelling will be used to track the movements of the cells during the phase of barrier-assembly. Finally, the model will be validated with respect to barrier function and cell-cell interactions. For this purpose, the microchannels will be perfused with fluorescent molecules, such as FITC-labelled dextran; the diffusion of the dye will be followed by live fluorescence microscopy. Immunofluorescence staining will be applied to image the proteins that are involved in the communication between pericytes and endothelial cells and to detect matrix components of the basement membrane. The migration of the cells towards different stimuli will be studied by introducing a gradient of chemokines (Figure 2C).
Conclusions and Relevance for 3R
In this project an in-vitro model of the endothelial barrier will be established. The system will embody the parameters that are of major importance in the three-dimensional environment of endothelial cells and pericytes. As such, it will afford a new insight into the phenotypic and functional characteristics of pericytes, which are believed to represent a physiological reservoir of MSCs. To date, most studies involving MSCs have relied on data that have been gleaned from monolayer-cultured cells, which has often hindered a translation of in-vitro findings into preclinically and clinically relevant information. Owing to the lack of suitable in-vitro models at the present time, data with more relevance to the physiological situation can be obtained only from in-vivo experiments. Our model may also be useful in the wider fields of angiogenesis, carcinogenesis and drug delivery. Hence, its development could help to numerically reduce animal experimentation across several research fields.
References
1. Armulik,A. et al. (2005) Circ. Res. 97, 512-523
2. Crisan,M. et al. (2008) Cell Stem Cell 3, 301-313
3. Bara, J et al. (2014) Stem Cells 32, 1713-23
4. Waters,J.P. et al. (2013) J. Vasc. Res. 50, 324-331
5. Morgan,J.P. et al. (2013) Nat. Protoc. 8, 1820-1836
6. Zheng,Y. et al. (2012) Proc. Natl. Acad. Sci. U. S. A 109, 9342-9347
Figures
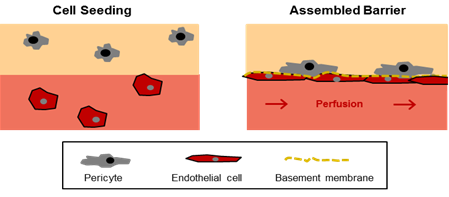
Figure 1: In-vitro model of the endothelial barrier. The model is comprised of two compartments (i) a hydrogel with encapsulated pericytes mimicking the perivascular tissue (beige) and (ii) a perfused microchannel containing endothelial cells (light red). In the assembled barrier the endothelial cells cover the microchannel, produce components of the basement membrane, and promote the migration of pericytes towards the endothelial layer.
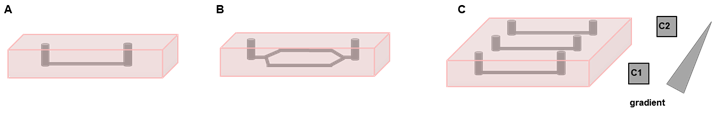
Figure 2: Various vessel configurations in the microfluidic system. A, B: Examples of microvessel geometries and designs; C: Parallel channels can be used to generate gradients of chemokines within the hydrogel.
| Letzte Änderung: 12.10.2018 |