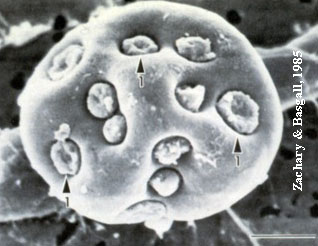
Figure 1: Electron microscopic picture of M. suis in close contact with the host cell (porcine erythrocyte; Zachary and Basgall, 1985).

Regina Hofmann-Lehmann
University of Zurich, Clinical Laboratory, Vetsuisse-Faculty, 8057 Zurich, Switzerland
rhofmann@vetclinics.uzh.ch
Keywords: pig; whole blood; mycoplasma; vaccination; veterinary disease; cell cultures: co-cultures; reduction; replacement
Duration: 3 years Project Completion: 2009
Background and Aim
Hemotrophic mycoplasmas (also named hemoplasmas) are the causative agents of infectious anemia in a wide range of domestic and wild animals (i.e. cat, swine, cattle, sheep, dog, lama, opossum). Current studies show that hemotrophic mycoplasmas are also apparent in primates (i.e. squirrel monkey, ‘Candidatus Mycoplasma kahanei’). The zoonotic relevance of hemoplasmas in humans is still unknown; the presence of human infections cannot be excluded. Hence, human infections with hemoplasmas are under investigation.
A major drawback for hemoplasma research is the unculturability of the agents. Thus, experimentally infected splenectomized animals are required for the propagation of hemoplasmas, e.g. as a source for microbiological, immunological and diagnostic analyses.
The aim of the research project is the establishment of an in vitro cultivation system for all hemotrophic mycoplasmas by using Mycoplasma suis as an appropriate prototype organism in order to replace the ethical questionable animal experiments.
Method and Results
Based on the recently established close phylogenetic relationship of hemoplasmas with the genus Mycoplasma we reason that hemoplasmas can be grown in pure culture by applying and diversifying proven culture techniques for mycoplasmas. Different culture approaches will be used to provide the microorganisms with the appropriate chemical and nutritional components of their natural environment, i.e. mammalian blood. Moreover, diffusion chamber methods will be applied in co-culture systems with eukaryotic cells and other fastidious mycoplasmal agents. Blood from experimentally infected pigs will be used as inoculum. The animals will be housed in the Clinic for Swines, Ludwig-Maximilians-University of Munich, Germany. The pigs need not to be infected within the scope of this submitted project but are a part of an approved experimental vaccination study. An M. suis-specific quantitative real-time PCR assay will be used to control the M. suis load of the inoculum and the growth and multiplication of M. suis in the different culture systems.
Conclusions and Relevance for 3R
The establishment of a hemoplasma in vitro cultivation system will replace all animal experiments which are currently necessary for the multiplication of these agents. New cultivation systems will open a wide range of possibilities:
- Clarification of pathogenetic phenomena in hemoplasma infections.
- Identification of virulence markers.
- Development and improvement of molecular and serological diagnostic assays.
- Realization of sequencing projects for hemoplasmas.
- Implementation of prophylactic measures since vaccine development and production is only possible from culture-derived hemoplasmas. Culture systems will allow attenuation and genetic manipulations of hemoplasma strains.
Figures

Figure 1: Electron microscopic picture of M. suis in close contact with the host cell (porcine erythrocyte; Zachary and Basgall, 1985).
Figure 2
Acridin orange stained blood smear of an experimentally M. suis infected pig (erythrocytes: labelled green; M. suis: labelled orange; Hoelzle, 2007).
Figure 3
M. suis infected pig showing typical clinical symptoms (acrocyanosis; Hoelzle, 2007).
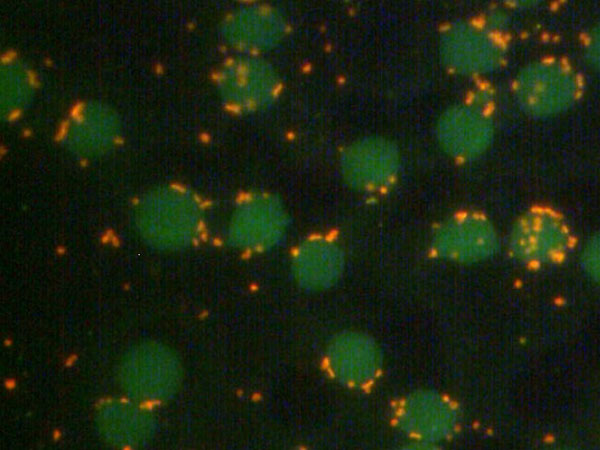
Figure 2
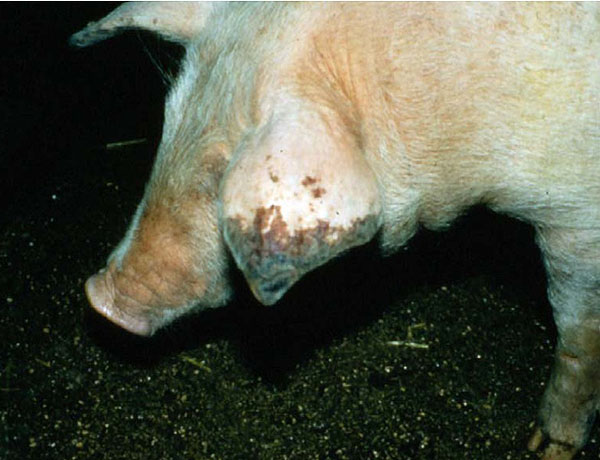
Figure 3