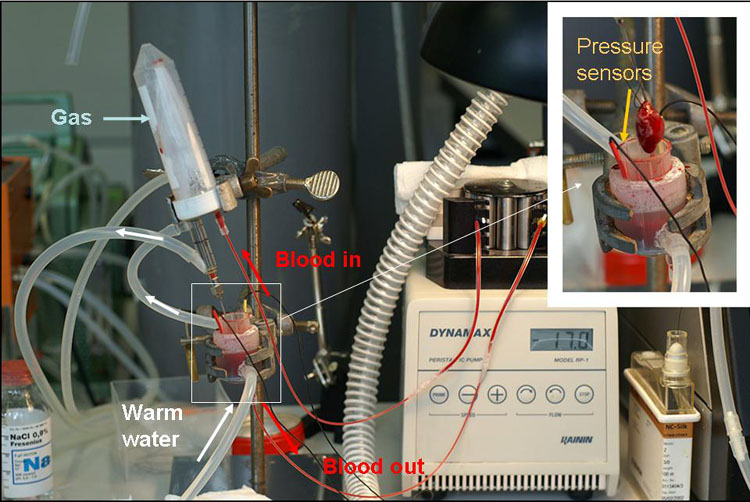
Figure 1: Experimental set-up

Anna Bogdanova and Johannes Vogel
University of Zurich, Institute of Veterinary Physiology, 8057 Zürich, Switzerland
annab@access.uzh.ch
Keywords: rat; heart; transplantation; ischemia; reduction; replacement
Duration: 2 years Project Completion: 2009
Background and Aim
The aim of this project is to develop a novel ex vivo model of rat heart perfused with autologous blood (see Fig 1). Responses of the isolated blood-perfused heart to reduction in oxygenation will be performed to characterise the model and define its potential to replace a common Tyrode-perfused Langendorff heart model and in vivo heterotopic heart transplantation model of ischemia-reperfusion injury.
Warm no-flow ischemia ultimately results in development of myocardial necrosis within 20-30 minutes after the interruption of blood circulation. In contrast, low-flow ischemia triggers a reversible reduction of contractile function in attempt to match ATP supply and consumption, a condition known as “hibernating myocardium”.
Mechanisms involved in a cross-talk between the myocardial contractile machinery, oxygen availability and metabolic pathways in hibernating heart are a matter of intensive investigation. According to our suggestion hypoxia is a major factor in tuning multiple responses which result in hibernation. Our new experimental model will allow precise control of blood oxygenation as well as blood flow rate thus mimicking hypoxia and low-flow ischemia conditions. Multiple parameters will be monitored in the heart tissue and in blood used for perfusion to evaluate its metabolic condition, redox state as well as the degree of myocardial stress and injury. The obtained results will then be compared with observations made on human patients with coronary artery disease resulting in acute or chronic low-flow myocardial ischemia.
Method and Results
Wistar male rats are anaesthetised with isofluorane, heparinised and 6-10 ml of blood are collected from the vena cava. The animals are decapitated and their heart harvested into ice-cold Tyrode buffer. After the circulation circuit is filled with blood the heart is mounted at the cannula and retrograde perfusion starts in a closed circuit with a hollow fiber oxygenator constructed by Johannes Vogel with blood oxygenated with a gas mixture containing O2, 5% CO2 and 75% N2 (see Fig 1). After 20 min of equilibration at pO2 20 kPa oxygen concentration, hypoxic perfusion is initiated by reducing the oxygen concentration in the gas phase of the oxygenator to 15, 10 or 5 %. During the perfusion heart rate is monitored using a piezoelectric pressure sensor.
After the perfusion protocol is completed the hearts are dismounted, and heart tissue harvested for the measurements of ion and water balance, metabolic and redox state markers.
Blood plasma samples are collected to measure (NO2-+ NO3-), BNP and TnT thus assessing mechanical stress and tissue damage. In a separate set of experiments, the use of glucose and fatty acids will be measured as a function of oxygen concentration in blood using autoradiography.
We are currently monitoring the changes in contractile rate, redox state as well as water and ion balance in the myocardial tissue of old and young animals exposed to hypoxic conditions. The preliminary data indicate that ageing makes the heart more vulnerable to the decrease in the blood oxygen pressure. Whereas decrease the hemoglobin oxygen saturation from 100 to 30 % triggers bradycardia and stunning in the aged heart, contractile rate of the young animal’s heart remains unaltered (Fig 2A and B). Hypoxia-induced decrease in contractile rate is the aged myocardium is followed by the oxidative stress, decrease in the hydrolytic activity of the Na/K ATPase and concomitant Na+ accumulation in the myocardial tissue. The observed changes in the heart function were not linked to the tissue ATP although basal ATP levels in the aged myocardium were significantly lower than those in the young animals.
Conclusions and Relevance for 3R
We have developed, optimised and validated the isolated autologous blood-perfused rat heart model. The heart sustains is spontaneous contractile activity for up to 3 h with the heart rate about 250-350 beats per minute and ECG parameters similar to those measured in an animal. Blood volume used for perfusion is known (5-7 ml) allowing precise adjustments of the concentration of any drug used. Temperature, blood perfusion rate and oxygenation state of blood are variables that may be precisely controlled as well. Blood shear-induced trauma and haemolysis present the major limitation of the model reducing experimentation time to 2 hours when blood is not replaced. This experimental model was tested when investigating the effects of deoxygenation on the heart rate, electric activity, ion/water balance, glucose metabolism and redox state. The obtained data indicate the ability of the heart to sense the changes in oxygen availability and rapidly adjust its pumping function as well as ATP generation and expenditure to the reduction in oxygen supply in order to avoid acute myocardial degeneration.
(see also 3R-INFO-BULLETIN Nr. 40)
References
1. Abbott CP, Dewitt CW, and Creech O, Jr. The Transplanted Rat Heart: Histologic and Electrocardiographic Changes. Transplantation 3: 432-445, 1965.
2. Langendorff O. Untersuchungen am überlebenden Säugetierherzen. Pflugers Arch 61: 291-332, 1898.
3. Ono K and Lindsey ES. Improved technique of heart transplantation in rats. The J Thorac Cardiovasc Surg 57: 225-229, 1969.
4. Sutherland FJ and Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613-627, 2000.
5. Segato Komniski M, Makhro A, Gassmann M, Bogdanova A, Vogel J. Isolated autologous blood-perfused heart: validation for rats and mice. submitted to the Am J Physiol 2009.
Figures

Figure 1: Experimental set-up
Figure 2A
Hypoxia-induced changes in heart rate in ex vivo perfused heart of a young animal
Figure 2B
Hypoxia-induced changes in heart rate in ex vivo perfused heart of an old animal
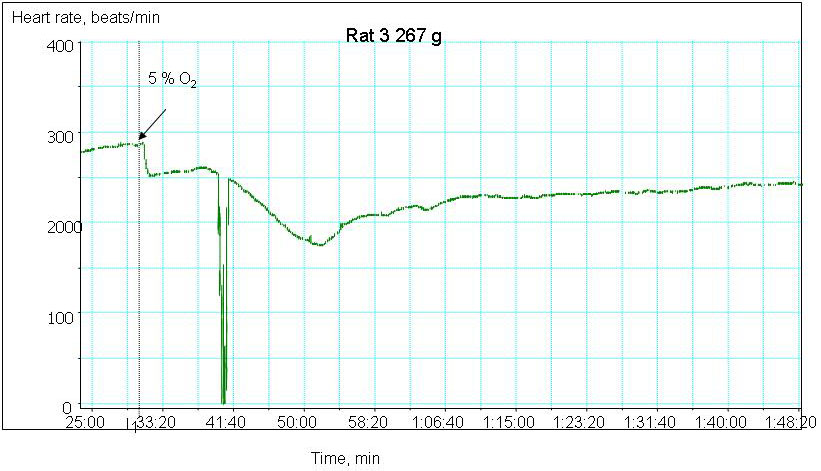
Figure 2
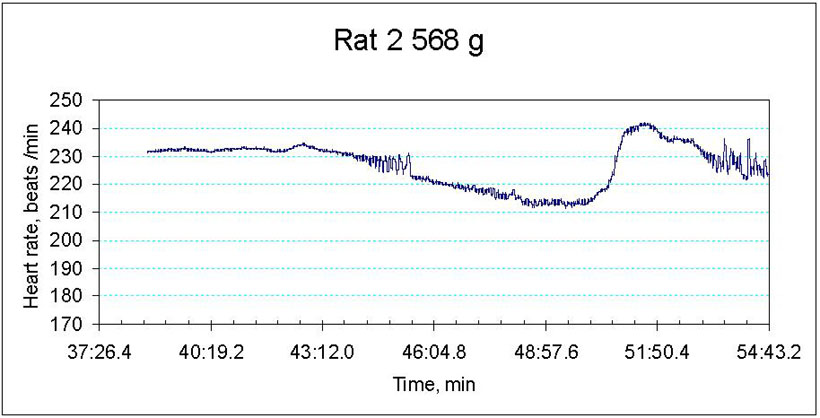
Figure 3