 |
3R-INFO-BULLETIN 49
June 2012
Authors

The project was carried out by a team of researchers (main investigator Sandra Hofer, PhD) lead by Stephen L. Leib (MD and professor) from the Neuroinfection Laboratory at the Department of Clinical Research, University of Bern, Switzerland. The research efforts of the team are focussed on infectious diseases of the brain with an emphasis on processes of neuronal injury and brain tissue repair in bacterial meningitis and in the development of viral encephalitis models.
Address:
Stephen L. Leib
stephen.leib@ifik.unibe.ch
Sandra Hofer
sandra.hofer@ifik.unibe.ch
Website: www.ifik.unibe.ch/content/forschung/group_leib_neuroinfection_laboratory_/index_ger.html
Neuroinfection Laboratory,
Institute for Infectious Diseases,
& Cluster for Regenerative Neuroscience
Departement for Clinical Research
University of Bern, Friedbühlstrasse 51
CH-3010 Bern,Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Bacterial Meningitis: Investigating Injury and Regenerative Therapy in vitro
Bacterial meningitis (BM) causes brain injury in the hippocampus, a structure critical for memory function. Due to the multifactorial pathogenesis involving the interplay between the brain cells, the bacteria and the host’s inflammatory reaction, the majority of research on meningitis is performed in animals. The present project (No. 103-06) supported by the 3R Research Foundation Switzerland is focused on the establishment of in vitro models that reproduce important pathophysiological processes of brain damage and tissue regeneration in infectious diseases of the brain.
Pathogenesis can be mimicked in cell cultures
Brain injury after bacterial meningitis is characterized by apoptosis of cells in the dentate gyrus of the hippocampus.1 Evidence from clinical and experimental studies suggests that both, the pathogen (bacterial components) and the host inflammatory reaction (e.g. TNF cytokine release and growth factor deprivation), contribute to the development of hippocampal injury.2 For this purpose we developed a cell-culture system with isolated hippocampus-derived neuronal stem/progenitor cells (NCP). It was possible to differentiate the cultured cells into defined developmental stages (stem cells, immature neurons and mature neurons, Fig 1A). Several days (1, 7, 14 and 21) after differentiation, they were challenged with different stimuli characteristic for bacterial meningitis (Fig 1B), i.e. growth factor deprivation and TNF-α (representing host reactions) or bacterial components (representing a pathogen). Subsequently programmed cell death (apoptosis), as observed in meningitis in the dentate gyrus, was documented by cleaved Caspase-3, Annexin-V or Apoptosis-Inducing Factor (Fig 1C). Thus, the newly established cell culture system recapitulated hippocampal neuronal differentiation in vitro and reflected the cellular response to typical insults of meningitis.
Which cell types are most susceptible to dying from bacterial meningitis?
Experimental studies showed that meningitis impairs hippocampal regeneration.3 This finding is reproduced in the newly established cell culture model where stem cells and immature neurons showed a higher incidence of apoptotic death than mature neurons after exposure to factors involved in the host pathogen interactions during meningitis (Fig 2 A, B, C). Our data support the view that stem cells and immature neurons are susceptible to apoptosis while mature neurons are more resistant to meningitis-induced death stimuli. This observation may help to understand why neurological deficits persist long after childhood bacterial meningitis and the hippocampal repair potential seems insufficient to compensate for the brain damage.4 The availability of this in vitro system leads to a substantial reduction of animal use as it provides for the screening of pathogenic mechanisms for their relevance prior to conducting large scale studies in vivo.
The in vitro findings were validated in vivo in an infant rat model of pneumococcal meningitis. The number of immature neurons and stem cells in the hippocampus in vivo was found to be proportional to the formation of neurospheres (free floating clusters of immature brain cells) from brain cells isolated from this brain structure. Accordingly, the capacity of hippocampal-derived cells to multiply and form neurospheres was reduced by approx. half in infant rats that survived pneumococcal meningitis compared to their uninfected littermates (Fig 3).
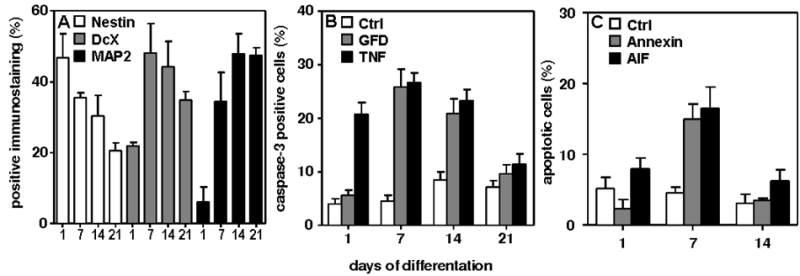
Fig 1
(A) Neuronal differentiation in vitro was documented by a gradual shift in the predominant staining pattern from Nestin (stem cells) at day 1 to Doublecortin (DcX; immature neurons) at 7-14 days to MAP2 (mature neurons) at 21 days (Ctrl=control).
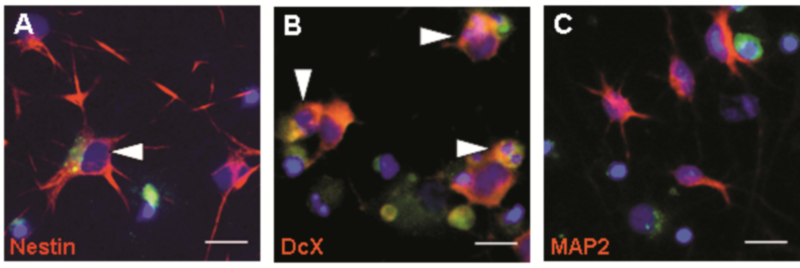
Fig 2
Immunocytochemical staining revealed that stem cells (A, red = positive for Nestin) and immature neurons (B, red = positive for Doublecortin) are susceptible to apoptosis while mature neurons (C, red = positive for Microtubule Associated Protein) are more resistant to death stimuli characteristic for bacterial meningitis Scale bar = 50 μM.
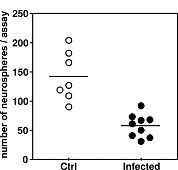
Fig 3
Significantly fewer neurospheres were obtained from the hippocampi of animals with meningitis (infected) compared to uninfected controls (Ctrl, p < 0.05).
From cells to tissue culture
Organotypic hippocampal slice cultures (OHC) retain the organisation of nervous tissue similar to that in vivo and offer a well-validated model for studying different stem cell grafts for their regenerative potential. In a previous study, the hippocampal apoptosis in the subgranular zone of the dentate gyrus was reproduced in vitro using the OHC system.5
An in vitro system of brain tissue regeneration
In the dentate gyrus of the hippocampus neurogenesis continuously occurs throughout life. A potentially attractive treatment option for support/reconstitution of neurogenesis after damage to the stem cell niche (e.g. in meningitis) is the delivery of regenerative cells to the site of cell loss. Currently, different sources of stem cells are being explored for potential use in repairing the brain. In general these explorative studies assessing the potential of different stem cell populations are carried out by performing in vivo studies. A large proportion of the cell types evaluated are not suitable for transplantation. To reduce and replace studies in animals, we established an in vitro pre-screening system of OHCs injured by challenge with bacterial components to evaluate different neuronal precursor cells (NPCs) for their repair potential in the injured hippocampus.6
Regeneration occurs in vitro
NPCs from the fetal hippocampus, expressing Green Fluorescence Protein (GFP), were grafted into the dentate gyrus of organotypic hippocampal slice cultures (OHC).. The migration and differentiation of grafted cells were examined on cryosections of intact OHCs and in those injured by challenge with bacterial components using immunofluorescence and histomorphometry (Fig 4 A, B). After transplantation NPCs migrated out of the dentate gyrus in intact OHC (Fig 4 A) whereas in injured OHC, NPCs migrated and integrated into the damaged dentate gyrus (Fig 4 B).
Comparable result with in vivo studies
Subsequently, the newly established in vitro system was validated by a limited in vivo study in an infant rat model of pneumococcal meningitis to demonstrate its appropriateness as an alternative to experiments in vivo. Application was done by stereotaxical transplantation of NPCs into the hilus region of intact and injured hippocampal dentate gyrus. Survival and integration were monitored by immunofluorescence and histomorphometry at 1, 2 and 4 weeks after transplantation (Fig 4 C,D).
Comparable result with in vivo studies
Subsequently, the newly established in vitro system was validated by a limited in vivo study in an infant rat model of pneumococcal meningitis to demonstrate its appropriateness as an alternative to experiments in vivo. Application was done by stereotaxical transplantation of NPCs into the hilus region of intact and injured hippocampal dentate gyrus. Survival and integration were monitored by immunofluorescence and histomorphometry at 1, 2 and 4 weeks after transplantation (Fig 4 C,D).
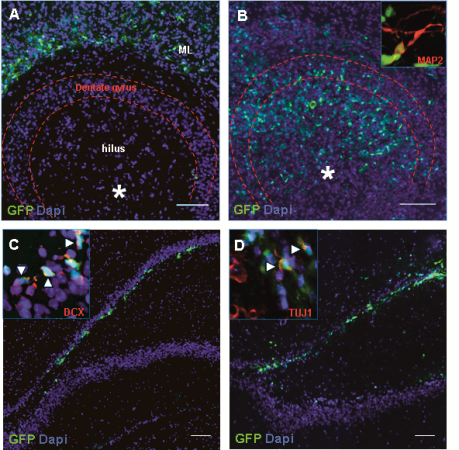
Fig 4
Transplantation of NPCs expressing Green Fluorescent Protein (GFP) in the hippocampus in vitro (A, B) and in vivo (C, D).
In vitro (A, B) migration of transplanted NPCs (green) after grafting into intact and pneumococci-injured organotypic hippocampal slice cultures (OHCs): A) The grafted NPCs derived from the hippocampus migrated from the area of engraftment (*) into the molecular layer structure (ML) outside of the dentate gyrus region in intact OHC, while B) they migrated to and integrated into to the area of cell loss in the dentate gyrus in injured OHC.
In vivo (C, D) NPCs (green) migrated and integrated into the damaged area of the hippocampus at 14 (C) and 28 (D) days after transplantation and expressed markers of neuronal development (DCX, insert C, and TUJ1, insert D). Scale bars 100 μm.
3R relevance
The experimental in vitro systems we established allow the reduction and/or replacement of the following in vivo investigations: i) testing of pathogenic hypothesis by in vitro screening of potential bacteria-derived mediators (e.g. bacterial cell wall components) and potential host factors (e.g. host inflammatory mediators), ii) assessment of the intrinsic properties of the different stages of cell differentiation which contribute to their selective vulnerability, and iii) evaluation of therapeutic approaches that involve the grafting of stem/progenitor cells into brain tissue. Thus, only approaches that proved successful in vitro would be considered for further evaluation in vivo.
PDF version of this Bulletin No. 49
References:
- Grandgirard D, Leib SL. Meningitis in neonates: bench to bedside. Clin Perinatol. 2010;37:655-676
- Grandgirard D, Leib SL. Strategies to prevent neuronal damage in paediatric bacterial meningitis. Curr Opin Pediatr. 2006;18:112-118
- Hofer S, Grandgirard D, Burri D et al. Bacterial meningitis impairs hippocampal neurogenesis. J Neuropathol Exp Neurol. 2011;70:890-899
- Grimwood K, Anderson P, Anderson V et al. Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Arch Dis Child. 2000;83:111-116
- Gianinazzi C, Grandgirard D, Simon F et al. Apoptosis of hippocampal neurons in organotypic slice culture models: direct effect of bacteria revisited. J Neuropathol Exp Neurol. 2004;63:610-617
- Hofer S, Magloire V, Streit J, Leib SL. Grafted neuronal precursor cells differentiate and integrate in injured hippocampus in experimental pneumococcal meningitis. Stem Cells. 2012;30:1206-1215
| Dernières modifications: 20.07.2012 |