
3R-INFO-BULLETIN 26
| | May 2004The Authors
The cell line was developed in the laboratory of Infectious Diseases, headed by Professor Dr. Regine Landmann, Division of Infectious Diseases, Department of Research, University Hospital Basel, Switzerland. The experimental part was carried out by the Dr. Daniel Dory.
Current addresses: Regine Landmann
regine.landmann@unibas.ch
Division of Infectious Dieseases
Department of Research
University Hospital Basel
Hebelstrasse 20
CH-4031 Basel
Switzerland Daniel Dory
d.dory@ploufragan.afssa.fr
AFSSA Ploufragan
Unite Genetique virale et Biosecurite
Zoopole Les Croix
BP 53
F-22440 PLOUFRAGAN
FRANCE
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Immune cells in the liver : The generation and use of a mouse Kupffer cell line
Investigations on the role of particular liver cell populations, in disease, are often associated with the use of a high number of animals. This is particularly true of Kupffer cells[*], the resident macrophages of the liver. These cells are difficult to study, because they are not homogenous, they survive and maintain their function in vitro, after isolation, only for a short period. The aim of the present successful projects (Nr. 63-97 and 73-00, supported by the 3R Research Foundation, Switzerland), has been to establish a Kupffer cell line, which would allow the molecular analysis of Kupffer cell function. Such an approach can replace a significant number of the widely performed animal experiments in the field of sepsis, transplantation and alcoholic liver disease. This line now allows a dissection of the complex interaction between different cell types in an intact liver. It has become possible to define the contribution of one particular Kupffer cell type, within the liver macrophages.
Impact of Kupffer cells in disease
Kupffer cells[*] play an important role in a number of diseases:
- Sepsis: During early sepsis, Kupffer cells - together with granulocytes, which accumulate in liver sinusoids - participate in bactericidal activity and release reactive oxygen and proteolytic products, which are a cause of liver injury.
- Alcohol induced injury: Due to alcohol induced,impaired gastrointestinal epithelial barrier function, Kupffer cells become exposed to increased levels of endotoxin, which cause a sustained production of inflammatory mediators. Consequently, Kupffer cell sensitivity to LPS is enhanced and contributes to chronic alcohol hepatitis.
- Hepatectomy: After partial hepatectomy for liver tumor resection, liver regenerates and all cells including Kupffer cells proliferate, these have a controversial effect on the growth of other liver cells.
- Liver transplantation: Kupffer cells interact with circulating T cells in the transplanted liver. Recent experiments in allograft models have shown, that Kupffer cells induce tolerance by suppressing T cell proliferation and induction of T cell apoptosis. Also, Kupffer cells isolated from an accepted allograft, prolong liver survival in a rejection model.
- Ischemia/reperfusion injury during liver surgery or transplantation: During liver surgery, perfusion may be interrupted by periods of ischemia; this causes irreparable damage to which Kupffer cells participate. Pre-exposure of the liver to transient ischemia, increases the tolerance to reperfusion, this process, is mainly mediated by Kupffer cells, which produce less pro-inflammatory cytokines and attract less granulocytes after ischemic pre-conditioning.
Together this demonstrates that it is highly relevant to know more concerning the function and role of this particular liver cell population.Why immortalized Kupffer cells?
Investigations concerning Kupffer cells are hampered, because in humans Kupffer cells are only accessible for immunohistochemical analysis, from biopsies or autopsies. From rats and mice they are difficult to isolate and after purification only approx. 5 million cells can be obtained from one mouse. Furthermore, these Kupffer cells are not homogeneous, form syncytia, rarely proliferate and survive for not more than 10 days. These reasons have made it mandatory to search for a way to immortalize Kupffer cells while preserving their original function.
How to generate a Kupffer cell line
Kupffer cells were isolated from H-2Kb-tsA58 transgenic mice, which stably express a thermolabile mutant of the Simian virus 40 (SV40) large tumor antigen under the control of a histocompatibility gene promoter (H-2Kb). Cells isolated from this mouse grow continuously at the permissive temperature of 33 °C, at which the mutant tsA58 is active, but don't grow, or there is less growth under the normal culture temperature of 37 °C. Growth is initiated by the incubation of cells with interferon-γ, which activates histocompatibility genes.
In the present project, we harvested Kupffer cells by collagenase perfusion of the liver, gradient centrifugation and subsequent counterflow centrifugation. Four lines were generated by culture at 33�C,in a medium containing interferon-γ and conditioned media from a hepatocyte and an endothelial cell line. In the absence of these paracrine growth factors, we observed a gradual loss of phenotype and secretory function.
One out of 4 clones (KC13-2) obtained by limiting dilution from the line, grew stably at 33�C without interferon-γ and also - although slower - at 37°C, which indicates an interferon-γ and temperature-independent regulation of SV40T Ag. Specific Kupffer cell characteristics of the KC13-2 clone were confirmed by comparing phenotypes and functions between peritoneal macrophages, primary isolated Kupffer cells and our clone.
We had to use 44 mice to establish and characterise the clonal line. In comparison: When working with primary Kupffer cells, a single experiment requires the livers of 10 mice and the variability between experiments is large.
What are these cells?
The generated cell line has been growing in culture, in a stable manner for more than 3 years . The cells of the clone, in contrast to the line and primary cells were uniform, survived detachment and could therefore be analyzed by flow cytometry. The KC13-2 clone, like the primary Kupffer cells, constitutively expressed a number of specific functions and structures. These incude: a) the classical macrophage enzymes non-specific esterase (Fig. 1a) and peroxidase; b) two macrophage-specific antigens of unknown function, MOMA-2 (Fig. 1b)
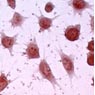
Fig. 1a: Enzymatic activity (Esterase) of Kupffer cell line
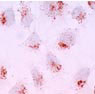
Fig. 1b: Phenotype (Moma-2 antigen) of Kupffer cell line
and BM8 (identical to the antigen known as F4/80); c) the pattern recognition receptors, which are activated by pathogen associated conserved molecules, including scavenger receptor A, CD14 and Toll-Like-Receptor 4/MD-2 (TLR4/MD-2), (Fig. 2a and 2b);
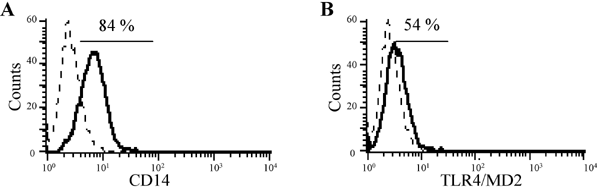
Fig. 2: Cell surface expression of pattern recognition receptors in the Kupffer cell line KC13-2, as measured by FACS analysis. The broken line indicates staining with the isotype control antibody, the black line indicates specific staining of (A) CD14 and (B) TLR4/MD-2; % positive cells are indicated.
d) the antigen presenting molecules MHC class I and II, CD40, CD80 and CD1d; e) Kupffer cells endocytosed Dextran-FITC, which is another characteristic of immature antigen presenting, dendritic cells; f) The lack of the phagosomal coat protein TACO, which is in all macrophages, except Kupffer cells; g) exhibition of CD14- and TLR4/MD2-independent, plasma-dependent lipopolysaccharide (LPS) binding. h) E. coli (Fig. 3a and 3b) and S. pneumoniae phagocytosis and LPS- and IFN-γ -induced NO production, but no TNF-α IL-6 or IL-10 release.
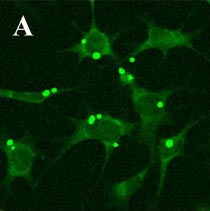
Fig. 3a: Phagocytosis of fluorescent E. coli by the Kupffer cell line KC13-2 (A) 1h at 37 °C.
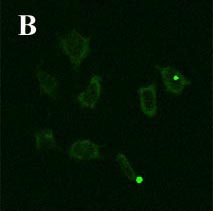
Fig. 3b: No phagocytosis after 1h at 4°C (control).
In summary the large size, surface marker expression and capacity to clear gram-negative and positive bacteria, but absence of cytokine release, indicates that the clone was derived from the periportal large Kupffer cell subpopulation.
A valuable tool
For the first time it has been possible to generate a stable, clonal Kupffer cell line representing a subpopulation within the Kupffer cells of rodent livers.
The applications for the use of the Kupffer cell line in disease models are multiple:
- Sepsis and alcoholic hepatitis: Signalling in Kupffer cells after stimulation with bacteria or bacterial products can be analyzed. Furthermore, virus or parasite entry into Kupffer cells and the immunological response can be followed.
- Liver regeneration: Soluble factors from the neighbouring cells (hepatocytes and endothelial cells) were required for maintenance of a differentiated phenotype in the Kupffer cell clone. This property may offer the possibility of identifying which factors modulate gene and protein expression in Kupffer cells and in studying interactions with other liver or blood cells in co-cultures.
- Liver transplantation: The set of antigen presenting molecules in the clone allows the study of the consequences of antigen presentation to MHC- and CD1-restricted T cells. Furthermore it will allow the investigation of the in vitro consequences of antigen presentation, such as T cell apoptosis e.g. in liver transplantation.
The clone allows molecular studies of the anti-infective and immune functions of Kupffer cells. It will reduce and replace studies with primary Kupffer cells obtained from mice.
However, control of in vitro derived knowledge has to be proven in selected cases, in an intact animal or directly in specific clinical applications.
Published updated version of this Bulletin 26/2007 (PDF)
References:
- Generation and functional characterization of a clonal murine periportal Kupffer cell line from H-2Kb-tsA58 mice J Leukocyte Biol. 74, 49-59, 2003 by D. Dory, M Letiembre, H. Echchanaouui, F. Ferracin, J. Pieters, Y. Adachi, S. Akashi and R. Landmann.
| [*] | What are Kupffer cells?Kupffer cells account for 80 to 90% of resident macrophages in the body, they constitute about 15% of the liver cells. Kupffer cells are located along the sinusoid, and exhibit different phenotypes and functional capacity in the periportal, intermediate and central area of the liver lobule (Fig. 4a). They have a life span of approximately 14 months and only a small fraction, amounting to 3% is responsible for cell population renewal in vivo. The principal role of Kupffer cells is phagocytosis and mediation of the innate immune response in the liver. Kupffer cells are able to remove from the blood any particulate or soluble gut-derived stimulus (Fig. 4b); after uptake they release reactive oxygen species, nitric oxide, cytokines and lipid mediators. The best known stimulus of Kupffer cells is the gram-negative,cell membrane constituent, Lipopolysaccharide (LPS) or endotoxin, which is a normal component of portal venous blood. Depending on the timing, frequency and intensity of endotoxin stimulation, sensitisation or tolerance to endotoxin results. Excessive stimulation of Kupffer cells leads to liver damage via release of cytokines and toxic oxygen products. 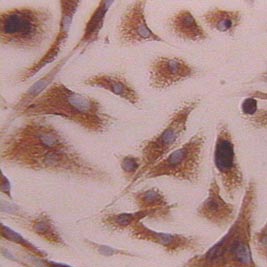
Fig. 4a: Primary Kupffer cell, cultured in vitro: Stained with a macrophage specific antibody. The cell size is not homogenous, because Kupffer cells from the periportal area are larger than those from intermediate or central lobular area.
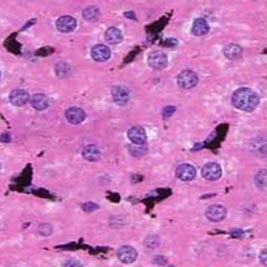
Fig. 4b: Histology of Liver Sinusoid : Kupffer cells are stained in black after ink uptake, they are interposed between hepatocytes.
|








