Figure 1: Tissue formation within the PET scaffold constructs after 4 weeks co-culture. There were greater number of chondrocytes and more tissue formation within the constructs after 4 weeks application of CCL (A) than within the control group (B).

Zhijie Luo, Bahaa Seedhom and Jennifer Kirkham
Leeds Dental Institute, Clarendon Way, Leeds LS9 9LU, UK
zhijie3@msn.com
Keywords: human; bone, cartilage; cell cultures: bioreactor; tissue engineering; reduction; replacement
Duration: 2 years Project Completion: 2011
Background and Aim
Tissue engineering offers potential solutions to the clinical challenges of cartilage repair and regeneration. Animal models are still widely used in evaluation of tissue engineering strategies for the repair of damaged human articular cartilage. However, there is no consensus on the most appropriate animal model and none of the species used replicate the anatomical, cellular and biomechanical properties of human articular cartilage. A further major problem is the poor integration of newly implanted engineered cartilage with adjacent healthy tissue (1, 2). Without full integration into native cartilage, the implants will experience abnormal mechanical stress during their post-surgical maturation and remodeling, potentially leading to further damage to both new and adjacent tissue and thereby limiting the efficacy of the repair. Application of mechanical stimulation to engineered tissue has been reported to enhance cartilaginous matrix formation and improve tissue growth (3-5). However, despite reports of improvements in the quality of in vitro engineered cartilage tissue, there is a lack of information about whether these ‘better quality’ engineered tissue constructs ever achieve successful lateral integration between repair tissue and adjacent cartilage after implantation.
A major aim of this study was to contribute to a reduction in animal experimentation by establishing a novel, holistic in vitro model to investigate tissue induction, remodelling and lateral integration to adjacent cartilage after cell-scaffold constructs are implanted in cartilage defect sites. This model would be used as an in vitro screening capability for newly developed technologies intended for cartilage repair, thereby not only reducing the numbers of animal procedures but also, in some cases, replacing entirely certain specific procedures that are currently carried out in vivo.
Method and Results
We developed a novel 3-D co-culture model for in-vitro evaluation of tissue-engineering techniques intended to provide constructs for cartilage repair. This model consists of a cartilage explant in the form of an annular disk, into which is inserted the engineered tissue construct (implant) intended for cartilage repair. Each construct, maintained sterile in culture, was exposed to cyclic compressive loads (CCL) over prolonged periods in our specially designed bioreactors. The preliminary loading regime used was within the physiological range at 0.2Mps, with a frequency of 0.5 Hz, applied for 30 minutes per day, 5 days per week for up to 9 consecutive weeks. Identical constructs were cultured in the bioreactor chamber without application of CCL and used as controls. Cell viability was assessed with confocal microscopy. Maturation of the implant tissue was evaluated biochemically and histologically. Mechanical properties of the constructs were measured in the form of their compressive modulus. The integration between engineered tissue and surrounding cartilage was also evaluated histologically.
Over 160 bovine cartilage explants were seeded on to scaffolds to provide constructs for testing in the above model. Two types of scaffolds were tested within the constructs:
(1) Non-absorbable scaffold made of non-woven filamentous polyester (PET). Chondrocytes from bovine knee cartilage were seeded onto PET scaffolds, encapsulated with 1.2% alginate gel and implanted into the central holes of cartilage rings and subjected to CCL at the above loading regime for 9 weeks. Identical constructs were cultured in the bioreactor chamber without application of CCL (controls). Constructs were investigated at 4 and 9 weeks.
(2) Bioabsorbable scaffold made of Type-VII low melting agarose (Sigma, St. Louis, MO) was dissolved in culture media, autoclaved, then mixed with chondrocytes at 37°C to a final gel concentration of 2% (w/v). The resulting constructs were implanted into cartilage rings and were subjected to CCL as described previously in (1) above.
Constructs formed from non-woven PET (1) showed greater amounts of tissue formation after CCL compared with non-loaded controls (Fig. 1) after 4 weeks of co-culture.

Figure 1: Tissue formation within the PET scaffold constructs after 4 weeks co-culture. There were greater number of chondrocytes and more tissue formation within the constructs after 4 weeks application of CCL (A) than within the control group (B).
Figure 2
Integration between the PET scaffold contructs and surrounding cartilage observed by confocal scanning microscopy showing more tissue integrated into surrounding cartilage in the CCL application group (A) than the Control group (B) after 4 weeks co-culture. Arrow shows the boundary between the implant and surrounding cartilage and there were gaps between the implant and cartilage at some sites. 95% cell viability within the implant was achieved on both CCL application group and control group.
Figure 3
GAG content of PET constructs ± CCL after 4 and 9 weeks in culture, expressed as total GAG per microgram of DNA. (n=6); * p<0.05; **p<0.01. CCL increased GAG synthesis at both time points.
Figure 4
Hydroxyproline content of PET constructs ± CCL, expressed as total hydroxyproline per microgram of DNA (n=6). No significant change after CCL application at both time points.
Figure 5
Mechanical testing of PET scaffold constructs after co-culture. Modulus was measured as tangent to the stress strain curves at strain values of 20 %. CCL significantly increased construct stiffness at week 9 (p,0.05).
Figure 6
Confocal microscopy of agarose constructs in co-culture at week 4, better integration was observed after CCL (A) compared with controls (B).
Figure 7
GAG synthesis within agrose constructs ± CCL after 4 and 8 weeks in culture, expressed as total GAG per microgram of DNA. (n=6): *, p<0.05. CCL increased GAG synthesis at both time points.
Figure 8
Hydroxyproline (HYP) content of agrose constructs ± CCL after 4 and 8 weeks in culture, expressed as total HYP per microgram of DNA (n=6). **P<0.01. CCL increased HYP at week 4.
Figure 9
Histological analysis of agarose-constructs at week 8 in co-culture: Safranin O stain (A,D,G,J), Alcian blue stain (B,E,H,K) and immunohistochemical stain of type II collagen (C,F,I,L) showing implant and surrounding cartilage (A-F) and constructs only (G-L). The expression of GAG and type II collagen was higher in the CCL group (A-C, G-I) compared with controls (D-F, J-L). Partial integration between implants and adjacent cartilage was observed (A-F).
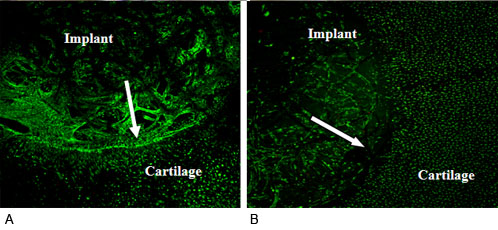
Figure 2
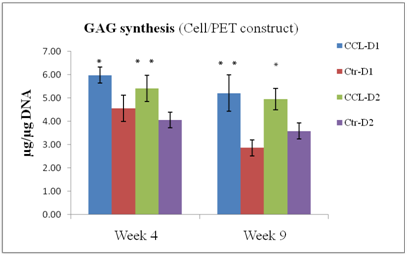
Figure 3
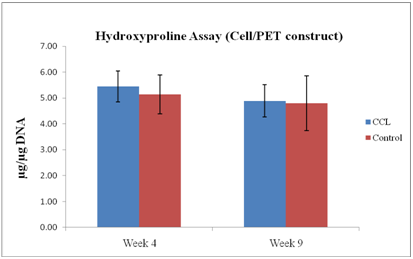
Figure 4
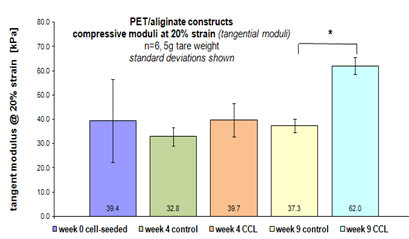
Figure 5
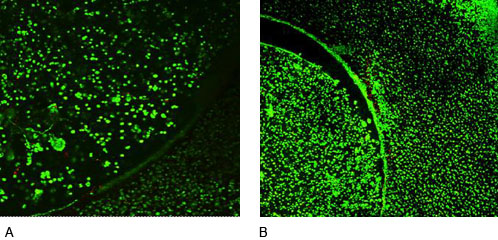
Figure 6
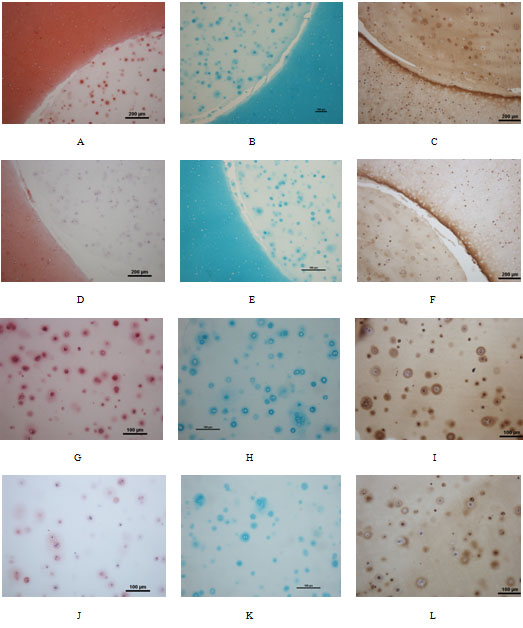
Figure 9
Conclusions and Relevance for 3R
The ultimate aim of this study was to develop an in vitro approach to screening various materials and constructs used in tissue engineering leading to the reduction in the number of animals used in pre-clinical trials of different implants and methods proposed for cartilage repair. We used a 3-D co-culture model, comprising a cartilage disc in the form of a ring, into which are placed different types of constructs intended for cartilage repair. These rings were then subjected to compressive cycling loading in sterile culture medium to investigate the effect of mechanical stimulus on the quality of repair tissue. The cyclic loading was applied in a bespoke bioreactor over a period of 9 weeks. Two types of constructs were tested.
Our data demonstrated that the quality of repair tissue was improved after CCL in terms of amount of tissue formed, biochemical and histological maturation, and the maintenance of cell phenotype. Differences in mechanical properties related to the two scaffolds used were also discerned; mechanical stimulus had a positive effect when PET scaffolds were used, improving construct mechanical properties but not when agarose gel scaffolds were employed.
These results indicate that our model has been successfully developed and it now available for future use as an effective tool to screen constructs that are made from different biomaterials and intended for cartilage repair. The screening of more promising constructs and materials at an early stage would reduce the number proceeding to further investigation in animal models and in turn would result in a substantial reduction of animals used in pre-clinical trials.
References
1. Archer C.W, Redman S, Khan I, Bishop J and Kirsty R (2006) Enhancing tissue integration in cartilage repair procedures. J. Anat. 209, 481-493.
2. Chang C.H, Kuo TF, Lin C. C, Chou C.H, Chen K.H, Lin F.H, Liu H.C. (2006) Tissue engineering-based cartilage repair with allogenous chondrocytes and gelatin-chondroitin-hyaluronan tri-copolymer scaffold: A procine model assessed at 18, 24, and 36 weeks. Biomaterials 27, 1876-1888.
3. Mauck R.L, Soltz M.A., Wang C.C, Wong D.D, Chao P.H, Valhmu W.B., Hung C.T, and Ateshian G.A. (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels J Biomech Eng 122, 252-60.
4. Mauck R.L, Nicoll S. B, Seyhan S. L, Ateshian G. A and Hung C. T. (2003) Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering Tissue Eng 9(4):597-611.
5. Miyanishi K, Trindade M.C, Lindsey D.P, Beaupre G.S, Carter D.R, Goodman S. B, Schurman D.J, Smith R.L. (2006). Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng 12(8): 2253-2262.
Figures
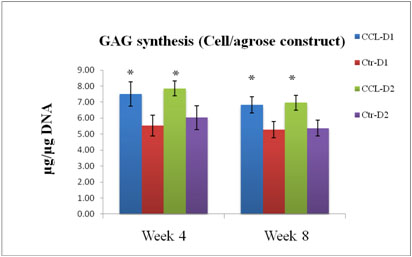
Figure 7
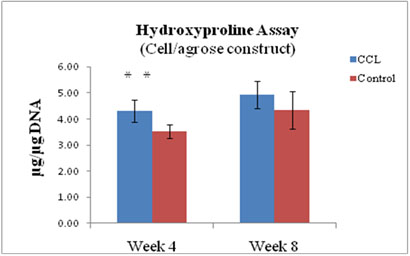
Figure 8