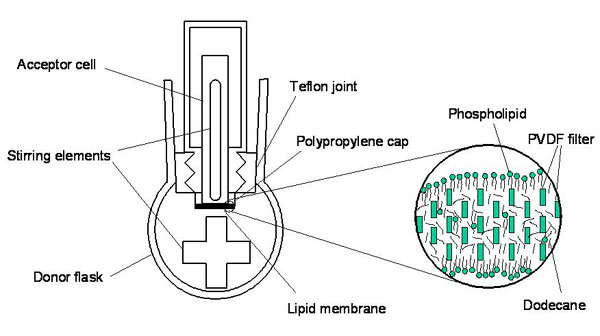
Figure 1: Experimental set-up of the PAMPA system

Beate Escher 1 and Jung-Hwan Kwon2
Dept. of Environmental Toxicology, EAWAG,CH-8600 Dübendorf, Switzerland.
present address: 1National Research Centre for Environmental Toxicology (EnTox), University of Queensland, 39 Kessels Rd, Coopers Plains, Qld 4108, Australia
2 Dept. Environmental Engineering
Ajou University
Wonchun-dong, Yeongtong-gu
Suwon, 443-749, South Korea, jhkwon@ajou.ac.kr
Keywords: fish; ecotoxicology; bioaccumulation; biochemical / analytical; in silico; reduction; replacement; bioaccumulation; drug screening
Duration: 2 years Project Completion: 2008
Background and Aim
The recent implementation of the new European chemicals legislation (REACh) has produced the requirement of a lot of additional testing, including bioaccumulation assessment in fish and other aquatic species. In addition continued activities in the field of Persistent Bioaccumulative and Toxic (PBT) assessment and the introduction of new Persistent Organic Polutants (POPs) to the Stockholm convention confirms the need for robust and efficient methods to assess the bioaccumulation of chemicals.
Bioconcentration testing is highly animal intensive. Thus alternative test methods have to be developed to reduce the number of test animals or to avoid the use of test animals by using alternative in-vitro test systems.
Bioaccumulation encompasses bioconcentration, i.e. the passive uptake (in fish via the gills), and biomagnification, i.e., the uptake via ingestion of contaminated food. Bioconcentration integrates the uptake, distribution and elimination of a substance due to water-borne exposure. In fish, bioaccumulation is typically dominated by bioconcentration due to the high surface area of the gill membranes. Metabolism decreases the Bioconcentraton Factor (BCF). Thus assessment models and in-vitro methods should account for metabolism. The in-vitro assay to be developed should therefore account for metabolic processes in fish.
The main goal of this project is the refinement of a new in-vitro method for evaluating bioconcentration kinetics in fish using the Parallel Artificial Membrane Permeability Assay (PAMPA) (Figure 1) to assess membrane permeation and membrane-water partitioning. With this information it should be possible to develop a prediction model to replace animal testing with the OECD 305 fish bioconcentration test. Low cost in-vitro tools are needed at the screening stage of assessment of bioaccumulation potential of new and existing chemicals because the number of chemical substances needs to be tested highly exceeds the capacity of in-vivo bioconcentration tests.
The second goal was to develop a prediction model to link the in-vitro results to in-vivo bioconcentration data in order to replace animal testing with the OECD 305 fish bioconcentration test.
Method and Results
The PAMPA system has been developed and widely used in pharmacokinetics research for assessing uptake of pharmaceuticals in the gastrointestinal tract and across other membrane barriers, such as epithelium cells. In a previous study we have used PAMPA for the first time to evaluate its potential for assessing passive absorption and elimination in small fish across fish gills. The initial work was very promising but the method still needed refinement to be applicable for hydrophobic pollutants of concern.
To overcome the difficulties associated with low aqueous solubility and high membrane affinity of highly hydrophobic chemicals, we measured the rate of permeation from the donor poly(dimethylsiloxane) (PDMS) disk to the acceptor PDMS disk through aqueous and PDMS membrane boundary layers and term the modified PAMPA system “PDMS-PAMPA”. In a second step, we combined the PDMS-PAMPA with an in-vitro metabolism assay.
For highly hydrophobic chemicals, it is very difficult to evaluate how fast they cross the membrane by analyzing aqueous concentration due to their low aqueous solubility and high partition coefficient between membrane and water. Therefore, 1 mm thick PDMS disks were placed in the donor and the acceptor solution to serve as a passive dosing/sampling phase. Twenty organic chemicals of which uptake and elimination rate constants were reported in literature for small fish were chosen for validation of the modified PAMPA [1].
For highly hydrophobic chemicals, membrane diffusion is much faster than aqueous boundary layer (ABL) diffusion both in-vivo and in-vitro. We developed an in-vivo to in-vitro extrapolation model to use the proposed PAMPA system for prediction. There are three factors affecting the performance of the in-vitro to in-vivo prediction model, the ABL thickness, the partition coefficient to the membrane surrogate phase (PDMS in this study [2]), and the surface-to-weight ratio of fish. An advantage of the presented PDMS-PAMPA system lies in the fact that the prediction model to relate the in-vitro results to in-vivo is not just a best-fit model but a theoretical model based on the underlying mechanistic processes. Using this model we obtained a good correlation with measured in-vivo elimination rate constants in fish, with the exception of metabolizable compound. For those we presently work in supplementing the experimental device by a fish S9 model system to include biotransformation processes in the in-vitro assay.
Conclusions and Relevance for 3R
The measured permeability of the 20 test chemicals was proportional to the passive elimination rate constant in fish and was used to predict the “minimum” in-vivo elimination rate constant. The in-vivo data were very close to predicted values except for a few polar chemicals and metabolically active chemicals, such as pyrene and benzo[a]pyrene. Thus, PDMS-PAMPA can be an appropriate in-vitro system for non-metabolizable chemicals.
If coupled with an in-vitro metabolism assay such as the fish S9 assay, which was also applied and refined in this project, a comprehensive in-vitro model is available for the prediction of bioconcentration potential of highly hydrophobic compounds.
Bioconcentration assessment in fish is highly animal and labor intensive. According to the OECD test guideline 305 „Bioconcentration: Flow-through Fish Test" (OECD, 1996) the test is performed during 28 days in two phases – uptake and depuration- with at least 9 sampling points using at least four fish each. Thus a minimum number of 40 fish is required for the determination of one BCF value for one fish species and one chemical. Considering that in the future more chemicals need to be assessed for their PBT properties (P = persistence, B = bioaccumulation, T = toxicity) due to the implementation of the new European Chemical’s legislation, there is an imperative need for alternative methods.
(see also 3R-INFO-BULLETIN Nr. 37)
References
[1] Kwon, J.-H., Escher, B. I. (2008), "A modified parallel artificial membrane permeability assay for evaluating bioconcentration of highly hydrophobic chemicals in fish", Environ. Sci. Technol., 42, 1787-1793.
[2] Kwon J-H, Wüthrich T, Mayer P, Escher BI. 2007. Partition coefficients of highly hydrophobic chemicals between polydimethylsiloxane (PDMS) and water. Anal Chem 79: 6816-6822
[3] Kwon, J.-H., Lee, S.-Y., Kang, H.-J., Mayer, P. and Escher, B.I. (2016) Including Bioconcentration Kinetics for the Prioritization and Interpretation of Regulatory Aquatic Toxicity Tests of Highly Hydrophobic Chemicals. Environmental Science & Technology 50(21), 12004-12011.
Figures

Figure 1: Experimental set-up of the PAMPA system