
3R-INFO-BULLETIN 9 - October 1996
| | The AuthorKarl Fent heads an ecotoxicology research group at the Swiss Federal Institute for Environmental Science and Technology (EAWAG), Dübendorf, and is a Privatdozent teaching undergraduate and graduate courses in ecotoxicology at the ETH and University of Zürich. After completing his Ph.D. in neurobiology he held a postdoctoral fellowship in toxicology with Professor G. Zbinden at the ETH and University of Zürich. From 1990-1991, he was a guest investigator at the Woods Hole Oceanographic Institution, USA. His research focuses on the effects of environmental chemicals on aquatic ecosystems. Since 8 years he works in the field of ecotoxicology at the EAWAG.  Fent K., PD Dr. Fent K., PD Dr.
EAWAG
Überlandstrasse 133
CH-8600 Dübendorf
Tel. +41 1 823 53 32
Fax +41 1 823 50 28
Email: Fent@eawag.ch |
Permanent Fish Cell Cultures as Novel Tools in Environmental Toxicology
In a 3R-Project K. Fent demonstrates that fish cell lines provide a novel tool in the environmental assessment of potentially hazardous chemicals
In vitro systems in ecotoxicology
The development of in vitro assays in ecotoxicology, or environmental toxicology, is needed for scientific, ethical, and economic reasons. About 80,000 chemicals (including 600 pesticides) are currently used, and more than 1,000 enter the market every year. Their ecotoxicological properties should be assessed prior to release into the environment. In vitro systems could play a valuable role in such assessments. Unfortunately, in vitro ecotoxicology tests are only recently gaining recognition from government, industry and the scientific community. As toxic effects are often species-specific, toxicity towards fish can only be assessed in fish-specific systems. Using permanent fish cell-lines, the goals of our research are (i) to develop novel in vitro assays for the assessment of environmental toxicity to fish, thereby reducing and replacing animal testing, and (ii) to demonstrate the general usefulness of this approach in basic and applied ecotoxicology.
Permanent fish hepatoma cells PLHC-1
Fish cell-line PLHC-1
The permanent fish cell-line PLHC-1, derived from a hepatocellular carcinoma in the topminnow Poeciliopis lucida, has several advantages over currently used fibroblast-like cells [1]. Firstly, they have metabolic activities, necessary to study the metabolism and the toxicity of environmental chemicals acting via metabolic activation. Secondly, PLHC-1 are easy to cultivate. Thirdly, they contain an aryl hydrocarbon receptor and possess inducible and stable cytochrome P450 (CYP) enzymes, which are important for detoxification of environmental chemicals. This cell culture system is ideally suited to assess the acute and chronic toxicity of chemicals, pollutants and environmental probes, and to derive structure-activity relationships within chemical classes.
Correlation of in vitro and in vivo toxicity
The cytotoxicity of more than 50 important environmental chemicals with various modes of action, including organotin (organic tin) compounds [2], chloro- and nitrophenols, sulfonic acids, and hormone-disrupting estrogenic chemicals (alkylphenols) have been assessed [3,4]. The neutral red assay based on the inhibition of neutral red uptake into lysosomes of viable cells and the tetrazolium salt reduction assay (based on mitochondrial metabolic function) were found to be most suitable tests, allowing rapid and reliable assessment.
An excellent quantitative correlation was found between the two assays. Organotin compounds were the most toxic, followed by higher substituted phenols including estrogenic nonylphenol, lower substituted phenols, and sulfonic acids. The in vitro results showed a trend similar to the in vivo acute toxicity in fish. Hence, acute fish toxicity of chemicals acting via different modes of action can be estimated in vitro. Permanent PLHC-1 cells are a promising tool in the toxicity screening and evaluation of chemicals prone to contaminate aquatic systems. Chronic effects such as induction of CYP1A demonstrated for PCB (polychlorobiphenyl) congeners and PAH (poly-aromatic hydrocarbons) can be estimated in environmental probes [5].
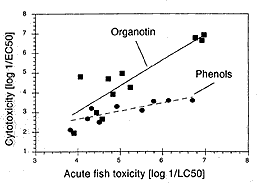
Relationship between in vitro cytotoxicity and acute in vivo toxicity in fish demonstrated with two compound groups.
Detection of hormone-disrupting chemicals
Considerable public and scientific concern has arisen over chemicals that act on hormone systems, because of their negative effects on reproduction. In vitro systems to determine the estrogenic activity of chemicals (e.g. permanent fish cell culture systems) are urgently needed. We evaluate the PLHC-1 for its potential in this respect. Preliminary results indicate that these cells possess estrogen receptor genes, and may possibly secrete vitellogenin. Vitellogenin is a precursor of the yolk protein in eggs; it is synthesized specifically in females and is regulated by estradiol. Hence, estrogenic chemicals that act via binding to the estrogen receptor may be detectable via this «biomarker». As we optimize cell culture conditions, we are currently focussing on this and other biomarkers for estrogenicity. As current techniques rely mainly on in vivo systems, a successful in vitro assay will help to reduce animal testing in product registration and safety.
Relevance
This fish cell culture system is a promising tool for basic and applied research, and for routine ecotoxicology tests. Our studies demonstrate its usefulness for rapid initial ecotoxicity screening and for environmental risk evaluation of chemicals. This will result in a reduction in animal testing in aquatic toxicology, where, in Switzerland alone, about 10,000 fish are currently used yearly for acute toxicity studies (OECD 203).
Published updated version of this Bulletin 9/2007 (PDF)
References
1. Ryan, J.A. and L.E. Hightower (1994). Evaluation of heavy-metal ion toxicity in fish cells using a combined stress protein and cytotoxicity assay. Environ. Tox. Chem. 13, 1231-1240.
2. Fent, K. (1996). Ecotoxicology of organotin compounds. Crit. Rev. Toxicol., 26, 1-117.
3. Brüschweiler, B.J., Würgler, F.E., and K. Fent (1995). Cytotoxicity in vitro of organotin compounds to fish hepatoma cells PLHC-1 (Poeciliopsis lucida). Aquat. Toxicol. 32, 143-160.
4. Fent, K. and J. Hunn. (1996). Cytotoxicity of organic environmental chemicals to fish liver cells (PLHC-1). Mar. Environ. Res. 42, 377-382.
5. Brüschweiler, B.J., Würgler, F.E. and K. Fent (1996). An ELISA for the determination of cytochrome P4501A in fish cell cultures. Environ. Toxicol. Chem. 15, 592-596.
Fent K., PD Dr.
