
3R-Project 135-13
In-vitro engineering of a human cell-based threedimensional dynamic model of atherosclerosis
Benedikt Weber and Arnold von Eckardstein
Swiss Center for Regenerative Medicine, University Hospital Zurich, Switzerland
Institute of Clinical Chemistry, University Hospital Zurich, Switzerland
benedikt.weber@usz.ch
Keywords: cardiology; cardiovascular; atherosclerosis
Duration: 3 years Project Completion: 2017
Background and Aim
Atherosclerosis is a chronic inflammatory disorder and underlies most cardiovascular diseases (Getz et al., 2012). According to the US National Center for Health Statistics(USNCHS) it is a leading cause of death in the Western world. Not surprising therefore, that research on atherosclerosis is becoming of increasing importance and more and more research resources are being invested in this particular field. Alongside this development, the use of animal models of the disease has increased during the past few decades. Owing largely to the relative ease with which genetic manipulations can be made in mice to the relatively short span of time over which atherosclerosis develops in this species, murine models are most extensively used at present. However, the development of atherosclerosis in mice differs from humans (Whitman, 2004). Consequently, larger animals have been implemented as models. These include rats, hamsters, guinea pigs, rabbits, birds, carnivors, dogs, swine, and various non-human primates (Getz et al., 2012). Although these models resemble more closely the human phenotype, substantial species-specific differences exist. Even when using dogs and non-human primates the data are not directly translatable to humans. Differences particularly in the lipoprotein profiles have been identified (Yin et al., 2012). The bioengineering of a human cell-based dynamic-artery model of atherosclerosis in-vitro could potentially help to reduce and replace many of the in-vivo experiments and afford an opportunity of performing experiments with human cells.
Method and Results
In progress (present status)
After the isolation of human vascular cells and the manufacture of PGA-P4HB-composite matrices, the bioengineered constructs will be fabricated using dynamic, pulsatile-flow bioreactor systems. After a quality assessment of the bioengineered arteries using Evans-Blue-based assays and the performance of biomechanical uniaxial tensile testings, LDL and monocytes will be isolated from human EDTA-treated blood, fluourescently-labeled and introduced into the circulation loop. Next, the formation of atherosclerotic lesions will be analyzed using immunohistochemistry, confocal microcoscopy, histology (after staining with Masson-Trichrome, Sudan Red or Red-Oil-O) and grating interferometry. Finally, the creation of transgenic human cell-based bioengineered grafts will be investigated after the deletion of specific genes in vascular cells / monocytes using the CompoZr® Zinc Finger Nuclease Technology (transgenic disease modeling platform).
Conclusions and Relevance for 3R
The bioengineering of a human cell-based dynamic-artery model of atherosclerosis could potentially hell to reduce and replace many of the in-vivo experiments that are currently performed with animals with animals such as mice, rabbits, pigs and non-human primates, and afford an opportunity of conducting investigations with human cells. Instead of executing (non-representative) experiments with animals, researchers could use the functional experimental human cell-based (representative) artery model to study the development of atherosclerosis and to investigate therapeutic options for its treatment.
References
Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 May;32(5):1104-15.
Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev. 2004 Feb;25(1):81-93.
Yin W, et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. 2012 Jan;53(1):51-65. doi:10.1194/jlr.M019927. Epub 2011 Oct 23.
Figures
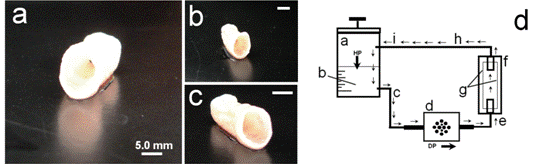
Figure 1: Tissue-engineered vascular grafts
(TEVG; a-c) after 2.5 weeks of in-vitro conditioning in a dynamic-flow bioreactor system (d). The basic set-up of the TEVG-system consists of a medium reservoir (A) filled with AFC-cell culture medium (B; HP = hydrostatic pressure). As a result of the pumping pressure, medium is sucked into the system (DP=dynamic-pressure generator) and via a connection tubing (E), is pressed into a container (F) containing the seeded TEVG-scaffold matrix (G). Next, the medium flows via further tubes (H-I) back to the reservoir and is replenished with nutrients. [Extracted from: Weber B., Tissue Eng Regen Med 2013]
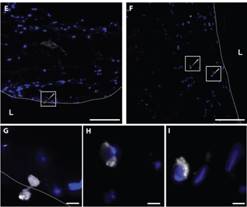
Figure 2: Monocyte transmigration in 3D artery-equivalents.
Monocyte adhesion and migration in the tissue were analyzed by the microscopic inspection of cryosections after pre-treatment with LDL´s. Microscopic observations revealed the adhesion of monocytes and their migration through the endothelium (dashed line) (E, G) as well as their subsequent accumulation in the tissue (F, H-I). Bars represent 200 µm (A-C, E-F) and 20 µm (G-I). [Extracted from: Robert J., Weber B., PLOS One 2013]


