
Extensive information about all the Foundation’s activities can be found on its website at www.forschung3r.ch. A survey of the Foundation’s website revealed that it received more visitors, thanks to the new format for the homepage. On average, 73 people visit the website every day. Questions are asked in all three languages featured on the website (English: 38%, German: 38%, French: 24%). Enquiries come from 150 different countries, with the most originating in the USA (35%), Switzerland (17.5%), Germany (10%), the UK (6.6%), France (5%), India (3.5%), Canada (3.3%), Ireland (2.7%) and Sweden (2.4%).
A total amount of Fr. 643,795.75 was paid out for 14 ongoing projects and 4 that were completed during 2007.
Three new projects were approved for funding during the past year for which a total of Fr. 336,191 was earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Standardization and Pre-validation of MucilAir: A novel in vitro cell model of the human airway epithelium for testing acute and chronic effects of chemical compounds (106/07) Dr. Song Huang, Epithelix Ltd., Plan-les-Ouates. The firm Epithelix has developed a cell culture system using human epithelial cells to test lung toxicity (MucilAir). The initial step is to pre-validate this in vitro process so that it can be considered by ECVAM for pan-European validation.
Evaluation of an in vitro model to identify host parameters associated with virulence of Toxoplasma gondii strains (107/07) Dr. Sushila D’Souza, Pasteur Institute, Brussels. The aim of this project is to determine the virulence of Toxoplasma gondii strains in cultures of human intestinal cells. At present the only way of determining virulence is by using a mouse test. This method could in the future be replaced by testing with cell cultures.
In vitro fish hepatocytes as source of metabolic clearance data in alternative approaches for the reduction or replacement of in vivo bioaccumulation testing with fish (108/07) Prof. Helmut Segner, Center for Fish and Wildlife Health, University of Berne. Predicting the bioaccumulation of pollutants in vitro is not reliable if the pollutants are metabolised. This project aims to standardise fish hepatocyte cultures to such an extent that they can be used for in vitro metabolisation of test substances. The metabolisation capacity of the cultures is characterised using 5 different reference substances.
A non-mammalian system to study bacterial infections (90/03) Prof. Pierre Cosson, University Medical Centre, Geneva. The virulence of bacteria was demonstrated using single-cell amoebae (Dictyostelium). It was observed that the virulence of selected bacteria was similar in amoebae and rodents. The same genes play a role in the immune system in amoebae and mammals. This method will make it possible to replace many of the highly stressful infection experiments using rodents.
The Transport of Active Substances in the Choroid Plexus (91/04) Prof. Gert Fricker, Ruprecht-Karls University, Heidelberg. A cell culture system was developed that accurately simulates the cell barrier in an intact organism (the choroid plexus epithelium). This method makes it possible to examine in vitro the processes involved in the exchange of substances between the brain/bone marrow fluid and the blood, and in many cases the use of laboratory animals will no longer be necessary.
Development of QSAR-Models for Classification and Prediction of Baseline Toxicity and of Uncoupling of Energy Transduction (95/05) Dr. Beate Escher, EAWAG, Dübendorf. The baseline or unspecific toxicity of environmental substances may be caused by a disturbance of energy transduction in cells and/or the disintegration of the membrane potential. It was possible to develop a quantitative structure-activity relationship (QSAR) for such substances. In this way the toxicity of these substances could be mathematically estimated and a large number of animal experiments could be avoided.
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
Exploring the natural anticoagulation by endothelial cells: A novel in vitro model (No. 34, January 2007) A descripton of how Prof. Robert Rieben and Dr. Yara Banz from the University of Berne (project 81/02) succeeded in obtaining the naturally anticoagulant effect of vascular endothelial cells in cell cultures. Consequently it is possible, for example, to identify substances that protect the endothelial cells from damage. Only such substances need then be tesed on laboratory animals.
From blood to brain and vice versa: Transport processes in choroid plexus can be studied in vitro (No. 35, May 2007) A description of how Prof. Gert Fricker and his team from the University of Heidelberg (project 91/04) succeeded in developing a cell culture system that accurately simulates the cell barrier in an intact organism (the choroid plexus epithelium). This method makes it possible to examine in vitro the exchange of substances between the brain/bone marrow fluid and the blood, and in many cases the use of laboratory animals is no longer necessary.
Press conference on 29 August 2007: Dr. Hugo Wick, Chairman of the Foundation, Christine Egerszegi, Speaker of the National Council in 2007 and Deputy Chair of the Foundation, Thomas Hartung, Director of ECVAM, Hans Wyss, Director of the Federal Veterinary Office and Thomas Cueni, Secretary General of Interpharma, each gave their impression of 20 years of pioneering promotion of research and dialogue in the service of animal protection and science. At the same time the new 3R brochure was presented.
New 3R brochure entitled “Good science with less animal experimentation”: This brochure sets out the 3R principles (replace, reduce, refine) from today’s point of view in a publication aimed at the general public. Its 36 pages cover the problems and limitations of replacing animal experimentation by alternative research methods, what has been achieved so far and possibilities and expectations for the future.
Scientific Jubilee Meeting, 3-4 September 2007: The 3R Research Foundation and the Swiss Laboratory Animal Science Association organised a scientific meeting at the Zurich-Irchel University under the slogan “3R = Better Science”. Over 400 visitors were recorded over the 2 days. In all 39 speakers presented papers and led workshops.
Special issue of ALTEX: This special English-language edition of ALTEX comprises reports of 20 successfully completed projects as well as the 17 ongoing ones. It also describes the sustainability of the completed projects and indicates what results can be expected in the future. Edited by Peter Maier (3R Research Foundation) and Franz P. Gruber (ALTEX).
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (Actelion Ltd, Merck Serono Ltd, Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, and the associated members Cilag Ltd and Vifor Ltd) and the Foundation for Animalfree Research (animal protection). It was entered in the commercial register on 18th August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods which avoid the use of animals, through grants for research projects. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer practical improvements vis-à-vis standard animal experimentation in line with the 3R motto Reduce, Refine, Replace.
A broad range of projects is sponsored on the condition that they are likely to reduce the number of animals used or the stress and/or pain suffered. Projects considered must be based on the Foundation’s three principles and are mainly in the bio-medical multidisciplinary field.
The Administrative Board of the Foundation is made up of nine members, three representing the Parliamentary Group for Animal Experimentation Questions (1 seat vacant), two representing animal protection, two from Interpharma and two from the Federal Veterinary Office. Current members are:
Dr. Hugo Wick, Basle (Chair until 31.12.2007)
Christine Egerszegi-Obrist, member of the National Council, Mellingen (Deputy Chair, Chair as from 1.1.2008)
Chantal Galladé, member of the National Council, Winterthur
Dr. Peter Bossard, Horw
Dr. Franz P. Gruber, Zurich
Dr. Peter Heer, Corporate Communications, F. Hoffmann-La Roche Ltd., Basle (until 31.12.2007)
Prof. Paul Herrling, Head of Research, Novartis International, Basle
Silvia Matile-Steiner, lawyer, F. Hoffmann-La Roche Ltd., Basle (as from 1.1.2008)
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld
Dr. Hans Wyss, Director of the Federal Veterinary Office, Berne-Liebefeld
Prof. Peter Maier, Uster (Chair)
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Prof. Kurt Bürki, Institute of Laboratory Animal Science, University of Zurich
Prof. Clemens A. Dahinden, Institute of Immunology and Allergology, University Hospital, Berne
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne
Dr. Kurt Lingenhöhl, Novartis Pharma AG, Basle
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld
Susanne Scheiwiller, B.Sc., Animalfree Research, Zurich
Prof. Peter Maier, Uster
KPMG AG, Gümligen-Berne
Federal Department of Home Affairs
In its twenty-first year of existence the Administrative Board met twice, namely in March and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2006, the Board addressed the following issues.
Research funds for 2007 were allotted to 14 projects already underway. In addition, 3 new projects were approved, while 11 applications were rejected. The Board also took note of the final assessment by the Evaluation Committee of 3 projects which had been completed in the previous years. In order to be able to ensure a solid financial basis for the future activities of the Foundation it was decided to ask Interpharma for a new, fifth promise of funding.
At the meeting in March 2007, discussions focused on the financial statements for 2006 as well as the celebrations to mark the 20th anniversary of the Foundation and the new 3R brochure. A further item on the agenda was the election of the Foundation’s officers for the period 2007 to 2010.
At its December meeting, the Board said goodbye to Dr. Hugo Wick, who had been Chairman since 1995 and was one of the founders, and elected Christine Egerszegi, a member of the Council of States, to succeed him. In addition, apart from the approval of new projects and final reports, the Board discussed financial issues in relation to the 2007 financial statements and the budget for 2008. The Scientific Adviser presented his report on the various events he had attended as the Foundation’s representative and the Board thanked him for his good work. They authorised him to organise a workshop in Basle in 2008 with guest speakers from the pharmaceutical industry as part of the ecopa “Start-up” project Scientific and technological issues in 3Rs: Alternative research in the process of drug development and union politics.
As far as concerns the activities surrounding the 20th anniversary of the Foundation, the Board was pleased to report a satisfactory outcome. While the press conference and the dinner on 29 August 2007 had attracted a relatively small number of people, the scientific meeting held on 3 and 4 September 2007 had been attended by over 400 people. The special edition of ALTEX, in which Prof. Maier and Dr. Gruber described 20 successfully completed projects as well as the 17 ongoing ones, also roused a good deal of interest in scientific circles. The highlight of public interest, however, was undoubtedly the new 3R brochure entitled „Good science with less animal experimentation”, which was sent out to some 10,000 people and extremely well received.
With the support of the scientific adviser, the Evaluation Committee held two meetings during the year, where in particular they assessed new applications and evaluated completed projects. The voluntary work of the members of the Evaluation Committee in this connection is much appreciated.
The Scientific Adviser's tasks included publishing the 3R Info Bulletin (as a brochure and on the Foundation’s website at www.forschung3r.ch), writing brief scientific reports in English which present the projects receiving funding and regularly updating the Foundation’s website. He was also kept busy monitoring the 3R Training Course internet learning programme. In addition, he spent much time – as always – advising applicants and project managers, obtaining intermediate reports, evaluating project outlines, dealing with enquiries and explaining why projects had been rejected. Finally, he represented the Foundation at several scientific meetings in Switzerland and abroad, namely as a member of the board at the Annual Meeting of the European Consensus Platform for 3R Alternatives to Animal Experimentation (http://www.ecopa.eu) in Brussels, as well as at the 6th World Congress on Alternatives and Animal Use in the Life Science, in Tokyo. As a member of the Advisory Board of the AcuteTox Consortium he attended a meeting in Stockholm. The Scientific Adviser also put a lot of work into helping to organise the scientific meeting at the Zurich-Irchel University, producing the special edition of ALTEX and helping to put together the new 3R brochure.

During the year 3 projects were completed (90/03, 91/04, 95/05). Together with those projects completed earlier (1-5/87, 6-15/88, 16/89, 17-20/90, 21-24/91, 25-42/92, 43-44/95, 45-55/96, 56-64/97, 65/98, 66-70/99, 71-75/00, 76-80/01, 81/02, 83/02, 85-88/03) this brings the total of finished projects to 89 out of 108.
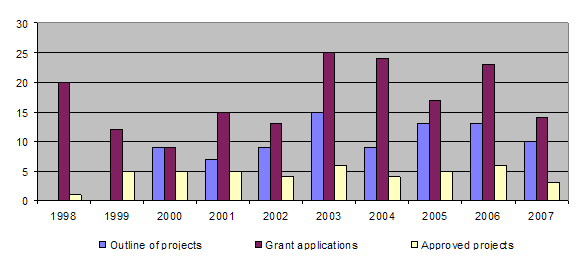
The bar-chart shows a downward trend in the number of applications received as well as an irregular pattern in the proportion approved. This can be explained by the fact that although in vitro projects are proposed, they are often not relevant to animal experimentation. If relevance to the 3R principles is to be taken in the strictest sense, it becomes increasingly difficult to design good projects. The long-term approval rate for applications is 30%.
The Foundation has set up the 3R Training Course internet learning programme to offer individual, specialised further training for people who carry out or supervise animal experiments. This course is available in German and English at http://3R-training.tierversuch.ch. Texts, images, links and documents provide visitors to the site with information on alternatives to animal experimentation. This course has been recognised officially as a further training course under the terms of the Federal Veterinary Office’s Ordinance of 12th October 1998 on the basic and further training of persons involved in animal experimentation (SR 455.171.2). Over the past year, 17 certificates were issued to people who passed the on-line examination.
The 3R Research Foundation and the Swiss Laboratory Animal Science Association (SGV) together with the Association for Training in Laboratory Animal Welfare and the Animal Keepers’and Technical Staff Interest Group, organised a scientific meeting on 3 and 4 September 2007 at the Zurich-Irchel University based around the slogan 3R = Better Science.
Over 400 people attended the 2-day meeting to hear 39 speakers who gave papers and led workshops. The 3R sessions, organised by the Foundation, drew 260 visitors. The 14 guest speakers succeeded in addressing the interdisciplinary topics in a fascinating way. The summaries of the papers have been published in a 70-page brochure. Special thanks are due to the Foundation’s Scientific Adviser, Prof. Peter Maier, who instigated and organised this successful event.
Prof. Peter Maier (3R Research Foundation) and Dr. Franz Gruber (editor of ALTEX ) put together a special edition of ALTEX (Alternatives to Animal Experimentation, Vol. 24, Special Issue 2007). This special edition comprises 104 pages in scientific English and describes 20 successfully completed projects as well as the 17 ongoing ones. The 20 projects selected, which have been completed during the Foundation’s 20-year existence, are proof of its sustainability. This edition is also used in the training course and can be ordered free-of-charge from the Foundation or downloaded in pdf form from our website.
“Good science with less animal experimentation” is the title of the new 36-page 3R brochure. The current stance on the 3Rs principle (replace, reduce, refine) promoted by the Foundation over the past 20 years is explained in general terms for interested lay readers. The brochure addresses the problems and limitations of replacing animal experiments with alternative research methods, describes what has been achieved so far and sets out future possibilities and expectations. The brochure is available in three languages (German, French and English) and has been put together in collaboration with Advocacy Ltd. and Continue Ltd, both based in Basle, by an editorial board consisting of Dr. Franz P. Gruber, Ursula Moser, Prof. Peter Maier, Dr. Heinz Müller, Adrian Heuss and Ernst P. Diener. It is also used in training courses for people who carry out experiments involving live animals. It can be ordered free-of-charge from the Foundation or downloaded in pdf form from our website.
All the members of the Board and the Evaluation Committee were willing to stand for re-election for the period 2007-2010. Dr. Hugo Wick and Dr. Peter Heer announced that they would be resigning at the end of the year. In December the Board wished them well and thanked them for all their good work on behalf of the Foundation. Dr. Wick, a former member of the National Council (CVP), was one of the instigators of the 3R Research Foundation and had been a member of the Board since it was set up in 1987. He took over as chairman in 1995. Dr. Heer joined the Board at the beginning of 1998 as a representative of Roche and Interpharma. He has been replaced by Silvia Matile-Steiner, who is a lawyer at F. Hoffmann-La Roche Ltd. No replacement for Dr. Wick from among parliamentarians has yet been found. As from 1 January 2008 the Board will be chaired by Christine Egerszegi, a member of the Council of States, who has been Deputy Chair until now.
| 108/07 | Prof. Dr. Helmut Segner Center for Fish and Wildlife Health, University of Berne In vitro fish hepatocytes as source of metabolic clearance data in alternative approaches for the reduction or replacement of in vivo bioaccumulation testing with fish |
| 107/07 | Dr. Sushila D’Souza Pasteur Institute of Brussels Evaluation of an in vitro model to identify host parameters associated with virulence of Toxoplasma gondii strains |
| 106/07 | Dr. Song Huang Epithelix Sàrl, Plan-les-Ouates Standardization and Pre-validation of MucilAir: A novel in vitro cell model of the human airway epithelium for testing acute and chronic effects of chemical compounds |
A complete list of projects with summaries of each can be found on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
The brief scientific project reports in English, which are updated once a year, also appear on the website and indicate that almost all projects have progressed well. The project managers’ reports tend increasingly to include helpful images. These reports published on the internet are much appreciated by those involved in the research projects as a platform for presenting their work. From the opposite point of view, this system also enables other researchers all over the world to discover new 3R methods without delay.
In 2007 two more new 3R-Info-Bulletins were published with a print-run of 1,000 copies each in English, and distributed among interested parties. The information bulletins are also published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
The latest 3R-Info bulletins are:
List of the other 3R-INFO BULLETINS
A total of some Fr. 812,200 was paid out for research in 2007 (Fr. 643,800 grants to research projects, Fr. 154,600 for activities in connection with the 20th anniversary celebrations, Fr. 8,200 for the internet training programme and Fr. 5,600 for participation in conferences). Some further Fr. 103,100 was spent on project monitoring and information, of which Fr. 13,300 was used for the 3R-Info-Bulletin and the Annual Report. A sum of Fr. 94,100 was spent on administration. Total expenditure therefore amounted to around Fr. 1,009,400.
Expenditure on current projects (Fr. 643,800) was some Fr. 34,000 under budget (Fr. 677,800); this was principally due to the fact that approximately Fr. 104,000 was paid out for two new projects, while some Fr. 110,000 earmarked for 3 projects was not used because the amounts budgeted for were not required in full. Of the 5% reserve (budgeted at Fr. 45,500) Fr. 18,000 was paid out upon the completion of certain projects. A sum of Fr. 5,600 was applied for in relation to participation in three conferences. The total of approximately Fr. 197,200 for project monitoring, information and administration was in line with budget (Fr. 198,900).
On the income side, Interpharma generously agreed to pay Fr. 55,328.50 towards the total expenditure of Fr. 246,965 in connection with the 20th anniversary celebrations, while the Federal Veterinary Office donated Fr. 12,000 towards the cost of distributing the 3R brochure. This left the Foundation with outgoings of Fr. 179,636.50 (3R brochure Fr. 119,216.70, printing Fr. 29,241.35, contribution towards distribution Fr. 3,000, ALTEX special issue Fr. 20,101.90, scientific meeting Fr. 8,076.55).
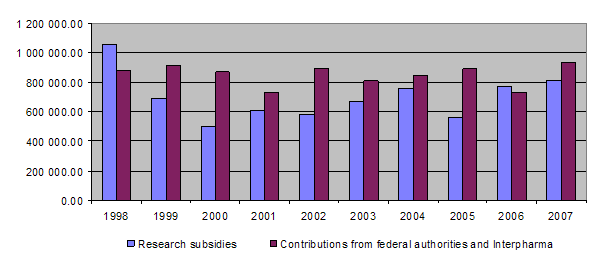
The equal financial commitment of the federal authorities and Interpharma represents the basic funding for the Foundation’s activities. With its fifth promise of funding on 20 December 2007, Interpharma once again declared its willingness to provide the Foundation with a total amount of Fr. 2,400,000. This will be transferred in annual instalments (maximum Fr. 600,000) on condition that the Foundation receives an equal amount from the Confederation. At the end of 2007 the Federal Veterinary Office promised an additional amount of Fr. 60,000, which was received at the beginning of 2008.
Thanks to the rise in interest rates, it was to the Foundation’s advantage to invest cash not required immediately in several different time deposits of up to 12 months, which resulted in interest earned of Fr. 10,500.
Total income was therefore around Fr. 944,500 (funding from the Confederation and Interpharma together being Fr. 930,000, interest earned amounting to Fr. 11,700, 3R training course exam fees yielding Fr. 1,700 and reimbursement of expenses in connection with the Ecopa survey Fr. 1,100) while total expenditure amounted to Fr. 1,009,400. This gives an excess of expenditure over income of around Fr. 64,900. The unused contributions item therefore fell from approximately Fr. 537,100 at the end of 2006 to Fr.472,200 at the end of 2007.
At the end of 2007 the total earmarked for projects approved by the Board but not yet paid out amounted to Fr. 908,044.55. This future liability is covered by Interpharma’s new promise of funding. Together with Interpharma’s funding which finished at the end of 2007, the Foundation’s credit with this institution amounted to Fr. 2,741,000 at the end of 2007.
The budget for 2008 includes around Fr. 623,000 for current projects and a maximum amount of Fr. 500,000 for new projects.
At the end of 2007 a total of Fr. 15,118,026.30 had been granted for projects and other subsidies, of which Fr. 14,209,981.75 has been paid out so far. Together the federal authorities and Interpharma have contributed Fr. 16,818,000 to the Foundation since 1987.
| Profit and loss account 2007 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 465 000.00 | ||
| Contribution from Interpharma | 465 000.00 | ||
| Total contributions | 930 000.00 | ||
| Interest on bank account | 11 676.10 | ||
| Research funds repaid | 0.00 | ||
| Other income | 2 862.00 | ||
| Total income | 944 538.10 | ||
Expenditure | |||
| Research grants | 812 165.35 | ||
| Project supervision and information | 103 102.65 | ||
| Administrative expenses | 94 122.95 | ||
| Total expenditure | 1 009 390.95 | ||
| Excess expenditure over income | - 64 852.85 | ||
| 944 538.10 | |||
| Balance as per 31st December 2007 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 467 740.84 | ||
| Accounts payable | 4 086.60 | ||
| Accounting apportionment assets | 10 930.80 | ||
Liabilities | |||
| Accounting apportionment liabilities | 9 473.00 | ||
| Unused project funds | |||
| Carried forward 1. 1. 2007 | 537 138.09 | ||
| expend. over income | - 64 852.85 | ||
| 472 285.24 | |||
| Capital of the Foundation | 1 000.00 | ||
| 482 758.24 | 482 758.24 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 908 044.55. | ||
Münsingen, 7th April 2008
3R RESEARCH FOUNDATION
President: signed C. Egerszegi
Secretary: signed E. Diener
As 3R Research Foundation’s auditors, KPMG AG in Gümligen-Berne has examined the books and the annual financial statements on the basis of current financial reporting standards and recommends that they be approved.