
3R-INFO-BULLETIN 56
| | February 2016Author
Dr. Sara L. Gonzalez Andino is a senior researcher at the Laboratory of Neural Microcircuitry at the EPFL. In 2004, she co-founded together with Rolando Grave de Peralta (co-applicant in this project) the Electrical Neuroimaging Group which specializes in the development of methods and models for the study of the bioelectromagnetic activity of the human body and the decoding of the information that is furnished by available electrophysiological signals (ENG). The ENG-group has developed several methods for the non-invasive estimation of neural activity from scalp EEG. Address: Dr. Sara L. Gonzalez Andino
sara.gonzalezandino@electrical-neuroimaging.ch Laboratory of Neural Microcircuitry (LNMC)
EPFL-SV-BMI-LNMC, Station 15
CH-1015 Lausanne
Switzerland
Editor
Ernst B. Hunziker, Scientific Adviser of the 3R Research Foundation |
Non-invasive electrical monitoring of the population spiking activity in the central nervous system
Diverse non-invasive neuroimaging modalities have been developed in the past. However, none of these offers the level of spatio-temporal resolution that can be achieved via invasive electrophysiological recordings in animals. These are capable of monitoring in real time the spiking activity of neuronal populations (multiunit activity, MUA) at the single-cell/population level. In project No. 119-10, we explored the possibility of estimating spiking activity from non-invasive scalp EEG measurements [1, 2] of the phase and amplitudes of local-field potentials (LFP). The latter have been reported to correlate with the spiking activity of neuronal populations [3].
Delta-Phase correlation with spiking activity is layer-dependent
Whittingstall and Logothetis [3] have shown that MUA in the primary visual cortex of monkeys can be predicted from increases in the power of the gamma-band activity that is recorded at the scalp surface (viz., in EEG) during the negative phase of the delta-band oscillations. A correlation between the phase of slow oscillations in the delta/theta band has been observed also in diverse structures in animals [4] and humans [5]. However, we discovered a clear counter-example to this rule (Figure 1) in an in-vitro model [6] of the slow wave oscillations (SWO) that are dominant during slow-wave sleep. In this model, correlations between the delta-phase/gamma power and MUA-activity of the visual cortex systematically depend upon the cortical layer in which the measurements are recorded.
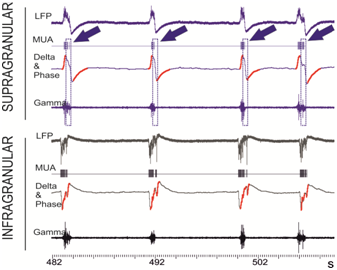
Fig 1.: Extracellular in-vitro recordings of LFP in the ferret visual cortex (10KHz) indicate that local MUA cannot be correctly predicted merely from the increases in the gamma-band power that occur during the negative phase of the delta band. Raw traces of the LFP that are recorded at supragranular (upper four: blue) and the infragranular (lower four: black) layers manifest reversed polarity during the SWO events. MUA (rows 2 and 6) and gamma-band (35-80Hz) oscillations (rows 4 and 8) are heightened in both, the infra and the supragranular layers during SWO. However, for the supragranular contacts, MUA and gamma-band oscillations are enhanced during the positive phase of the delta oscillations (see boxed areas which are indicated with arrows). The negative phase of delta oscillations (red) overlays the delta-band (0-4Hz) filtered LFP (rows 3 and 7).
Consequently, the sign of the phase of delta oscillations does not afford unequivocal information appertaining to the spiking activity of neural populations. It cannot be therefore used to rigorously constraint the mathematical problem as to lead to unique non-invasive estimates of MUA.
Quasistatic ohmic models fail to adequately describe the propagation of SWO in cortical tissue
Models underlying the non-invasive estimation of neural activity from scalp EEG rely on the validity of the quasistatic ohmic approximation (QSOA) of Maxwell equations. According to this approximation, which is extensively applied in clinical and experimental neuroscience, and which is used as the basis for the model underlying our non-invasive LFP estimates [1], the electric field should travel instantaneously from the sources to the sensors. Motivated by recent experimental results demonstrating that the propagation speed of epileptiform activity within hippocampal tissue [7] is far too low (~0.1m/s) to justify quasistatics, we critically re-examined this assumption. To do so, we studied the propagation speeds of SWOs in slices of the ferret visual cortex after isolating the synaptic from the electromagnetic transmission. Contrary to the damped, undistorted instantaneous propagation of slow waves, we observed substantial propagation delays that are incompatible with current modelling assumptions (Figure 2).
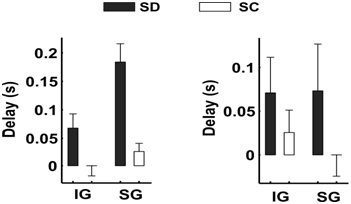
Fig 2.: Propagation delays of the SWO between contacts that are synaptically disconnected (SD: black bars) and synaptically connected (SC: white bars). Results reflect average values for 30 experiments. SWOs were evoked by local releases of glutamate at infra (IG) and supragranular (SG) contacts. Delays were defined as the time taken by the SWO to travel to nearby contacts (distances less than 1mm) in the recording grid. A cut was made on the slice to investigate propagation delays in the absence of synaptic connections, where propagation of the SWO is expected to reflect the propagation speed of the electric field. Mean propagation delays between SD-contacts were in the order of 70ms, which corresponds to a propagation speed of about 17mm/s.
In the light of this evidence, we were forced to develop a new model to estimate the LFP from scalp-recorded EEG. Interestingly, we were able to show that the irrotational source model from ELECTRA holds true after dropping the quasistatic approximation [8]. Moreover, a new non-quasistatic estimate of the current source density (CSD) was developed. The experimental validation of these models is currently underway.
Relevance for 3R
An accurate estimation of the spiking activity of neural populations from non-invasive scalp-based measurements of the electric activity of the brain (EEG) might substantially reduce the use of neural implants and the dangers that are associated with brain surgery in animals and more particularly in primates. Further developments in non-invasive approaches are expected in the long-term to replace invasive chronic LFP-recordings in animals which is in line with 3R-philosophy.
PDF version of this Bulletin No. 56
References:
- Grave de Peralta Menendez, R., et al., Imaging the electrical activity of the brain: ELECTRA. Hum Brain Mapp, 2000. 9(1): p. 1-12.
- Grave de Peralta Menendez, R., et al., Electrical neuroimaging based on biophysical constraints. NeuroImage, 2004. 21(2): p. 527-539.
- Whittingstall, K. and N.K. Logothetis, Frequency-Band Coupling in Surface EEG Reflects Spiking Activity in Monkey Visual Cortex. Neuron, 2009. 64(2): p. 281-289.
- Skaggs, W.E., et al., Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus, 1996. 6(2): p. 149-172.
- Jacobs, J., et al., Brain Oscillations Control Timing of Single-Neuron Activity in Humans. J. Neurosci., 2007. 27(14): p. 3839-3844.
- Sanchez-Vives, M.V., Spontaneous rhythmic activity in the adult cerebral cortex in vitro, in Isolated Central Nervous System Circuits, H. Press, Editor. 2012. p. 263-284.
- Zhang, M., et al., Propagation of Epileptiform Activity Can Be Independent of Synaptic Transmission, Gap Junctions, or Diffusion and Is Consistent with Electrical Field Transmission. The Journal of Neuroscience, 2014. 34(4): p. 1409-1419.
- Grave de Peralta Menendez, R. and S. Gonzalez Andino, Electrical Neuroimaging with Irrotational Sources. Computational and Mathematical Methods in Medicine, 2015. 2015: p. 8.



