
3R-INFO-BULLETIN 24
| | September 2003The Authors 
The project was carried out by Prof. Andrew Hemphill and Dr. Nathalie Vonlaufen at
the Institute of Parasitology, University of Bern, Switzerland*.
Andrew Hemphill is head of a research group at Institute of Parasitology at the University of Bern, Switzerland. He examines various aspects of N. caninum cell biology, including interactions between host and parasite, and the mechanism of entry of the parasite into host cells. In his group they also identify and characterise potential vaccines against N. caninum, and investigate interesting new chemotherapeutic agents (potential anti-protozoal drugs). Current address: Andrew Hemphill
Andrew.hemphill@ipa.unibe.ch
Institute of Parasitology
University of Bern
Länggass-Strasse 122
CH-3012 Bern
Switzerland *Collaborators in this project were Dr. Reto Caldelari and PD Dr. Eliane
Müller, (CELLnTEC advanced cell systems, Institute of Animal Pathology,
Bern), and Profs. Stephen Leib and Norbert Müller (Institutes of Infectious
Diseases and Parasitology, respectively, University of Berne, Switzerland).
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Generation of parasite cysts in cultured cells instead of living animals.
The protozoan parasite Neospora caninum is the most important infectious cause of abortion in cattle worldwide (Refs 1, 2)[*]. The bradyzoite stage of this parasite is infective by oral ingestion. Studies on stage conversion or anti-protozoal drugs have relied so far on animal models to produce bradyzoites. In the present project (Nr. 72-00), a method was developed to generate N. caninum bradyzoites in cell culture. This efficient and easy-to-handle in vitro alternative to producing N. caninum tissue cysts in animals will reduce the number of animals used in research on Neospora caninum and neosposrosis including the screen for anti-protozoal drugs.
Dog-Cattle and Tachyzoite-Bradyzoite stages
N. caninum can live in two different forms within the cells of dogs and cattle: the actively proliferating, disease-causing, tachyzoite stage, and the bradyzoite stage that lies dormant in tissue cysts (Fig.1). A third form of the parasite, the oocyst stage, is a sexually produced and resistant form responsible for the transfer of the infection from animal to animal (ie. this form exists external to the host). The life cycle of the parasite has been partially elucidated (Fig.1). Most studies on N. caninum carried out to date have relied largely on animal models. So far it has only been possible to culture the rapidly proliferating tachyzoite stage in cell lines and primary monolayer cell culture systems.
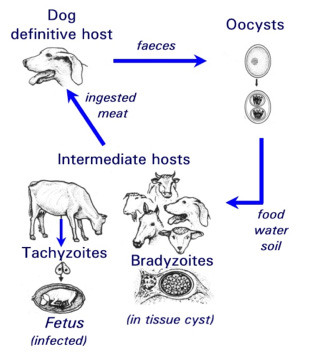 Fig. 1: Life cycle of the parasite Neospora caninum.
Fig. 1: Life cycle of the parasite Neospora caninum.
Bradyzoites a relevant target?
Until recently, research on the bradyzoite stage of N. caninum has been largely neglected. However there are two situations where bradyzoites represent a danger. Firstly, if the infected host becomes partially immuno-compromised, as occurs during pregnancy, bradyzoites are reactivated and transform to the rapidly proliferating tachyzoite stage. This can lead to an infection of the placental tissue and the fetus, resulting in acute fetal neosporosis and abortion. Secondly, in dogs that consume meat infected with N. caninum bradyzoites, the parasite can infect the intestine and undergoes sexual reproduction. This produces oocysts that are excreted in the faeces and contaminate soil, water and animal fodder. Thus, the epidemiology and disease-causing potential of N. caninum is indirectly influenced by the occurrence of bradyzoites within infected hosts, and strategies to prevent neosporosis must take into account all stages of the parasite, including bradyzoites.
The realization of the importance of this stage has led to the development of a mouse model to produce N. caninum bradyzoites. Usually artificially immuno-compromised mice are used, with obvious unpleasant side effects for these animals. In addition, the method is not very reliable, and requires large numbers of animals. A reliable in vitro culture model to generate N. caninum bradyzoites would therefore offer several significant advantages.
Hitting the jackpot: In vitro tachyzoite-to bradyzoite stage conversion in murine epidermal keratinocytes
Experiments to achieve N. caninum stage conversion in cell culture are based on the rationale that the parasite converts from the tachyzoite to the bradyzoite stage when it is under stress. Under conditions of stress, the parasite stops proliferating, and expresses bradyzoite-specific antigens that lead to its encapsulation into a tissue cyst. The techniques described earlier to achieve in vitro bradyzoite differentiation of the closely related apicomplexan Toxoplasma gondii were not effective for N. caninum (Ref. 4) , thus other ways had to be elucidated. Initial studies involved a number of different in vitro culture stress conditions, such as alterations in temperature and CO2-concentrations, pH-variation, exposure to immuno-modulators such as IFN-gamma and TNF-alpha, anti-parasite antisera and nitric oxide treatment. In a second phase, a number of parasite isolates and a variety of host cell systems were tested, including organotypic cultures of rat neural tissue (this is important because it involves not a cell line but a rat neural tissue as an alternative approach) (Ref. 3). Finally, a reliable and efficient method to obtain N. caninum differentiation from the proliferative to the cystic stage was established (Ref. 4): murine epidermal keratinocytes were infected with tachyzoites of the Nc-Liverpool isolate, and the infected cultures were treated with a high dose (70 μM) of a nitric oxide donor (sodium nitroprusside (SNP)) for up to 8 days. Medium changes had to be performed each day during the culture period. As assessed by Neospora-specific quantitative real-time PCR (Ref. 5), this treatment significantly reduced parasite proliferation. An upregulation of the expression of bradyzoite-specific antigens was detectable by immunofluorescence using antibodies directed against the bradyzoite-specific antigen NcBAG1, and in parallel, a downregulation of the expression of tachyzoite-specific immunodominant antigens such as NcSAG1 or NcSRS2 (Fig. 2). After 8 days of SNP treatment, the cyst wall antigen NcCC2 was secreted by the parasites and became associated with the cyst periphery (Fig. 3). Transmission electron microscopy revealed that these parasites were always located within intracellular compartments called parasitophorous vacuoles, and that the majority of the parasites within intracellular vacuoles were in the process of forming a distinct cyst wall, typical for the bradyzoite stages Fig. 4)
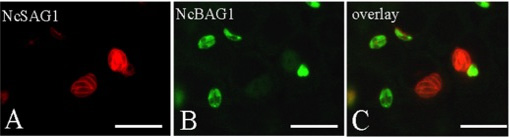
Fig. 2: In vitro stage conversion of N. caninum investigated by double-immunofluorescence. Note the downregulation of expression of the tachyzoite antigen NcSAG1 in those parasites which exhibit expression of the bradyzoite antigen NcBAG1. Bars = 20 μm
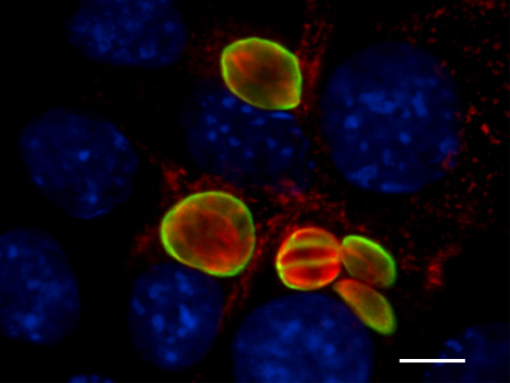
Fig. 3: Effects of SNP treatment (8 days) on expression and localization of the cyst wall antigen NcCC2 (green) with the periphery of the bradyzoite-containing vacuoles, which is indicative for cyst wall formation, Parasites are labelled in red, host cell nuclei are blue. Bar = 12 μm
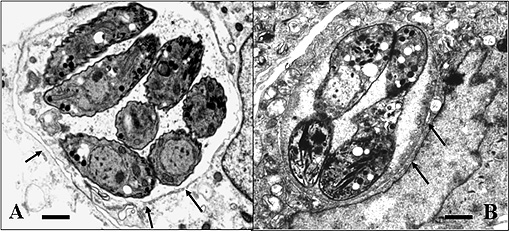
Fig. 4: Electronmicroscopical comparison of N. caninum tachyzoites (A) and bradyzoites (B) cultured in vitro. Note that both stages are located within an intracellular vacuole, but bradyzoite vacuoles (B) exhibit a defined cyst wall (thick dark structure between the arrows), which is lacking in tachyzoites (A) (cf. arrows). Bars = 1,3μm in (A) and 0.8 μm in (B).
Mass production: Neospora stage conversion in Vero cells
Although the keratinocyte culture system was efficient, with about 60% of parasitophorous vacuoles containing parasites expressing the bradyzoite marker NcBAG1 and therefore designated as bradyzoites, the system was not practical to produce large amounts of N. caninum bradyzoites for biochemical, molecular or functional infection studies. A modified procedure was therefore developed which enabled us to produce, far more economically, N. caninum bradyzoites in African green monkey kidney (Vero) cells (Ref. 6). In this system, stage conversion occurs at a much lower concentration of SNP (17 μM), a concentration which is tolerated by Vero cells for a limited time, but still leads to bradyzoite differentiation in about 60% of the parasites (Fig. 5). Additionally, a protocol, based on selective physical rupture of infected host cells and separation of host-components and intact bradyzoites by size exclusion chromatography, was elaborated to purify these bradyzoites from cell cultures. This has now opened avenues to dissect this important life cycle stage of the parasite at the biochemical and molecular level.
The 3Rs go together with higher scientific output.
The goals relevant for 3R have been achieved in that we provide a promising, efficient and easy-to-handle in vitro alternative to producing N. caninum tissue cysts in animals. This method will reduce the number of animals used in research on Neospora caninum and neosposrosis.
The N. caninum bradyzoite tissue cyst model is currently used to:
- screen anti-protozoal drugs (first round in vitro screening)
- identify stage-specifically expressed bradyzoite antigens to (i) improve immunodiagnosis and (ii) identify immuno-protective vaccines for dogs and cattle.
- experimentally infect dogs, (i) in order to verify the biological functionality of in vitro generated tisue cysts, and (ii) to produce oocysts to develop diagnostic tests and assess immunological responses in dogs and cattle
- investigate the cell biology of tachyzoite-to-bradyzoite conversion
- analyse the reactivation process in vitro (bradyzoite-to-tachyzoite reconversion)
Published updated version of this Bulletin 24/2007 (PDF)
References:
- Hemphill A. and Gottstein B. 2000. A European perspective on Neospora caninum. Int. J. Parasitol 30, 877-924.
- Hemphill A. 1999. The host-parasite relationship in neosporosis. Adv. Parasitol. 43, 47-104.
- Vonlaufen N., Gianinazzi G., Müller N, Simon F., Björkman C., Jungi T. W., Leib S. L and Hemphill A. 2002. Infection of organotypic slice cultures from rat central nervous tissue with Neospora caninum: an alternative approach to study host-parasite interactions. Int. J. Parasitol. 32, 533-542.
- Vonlaufen, N., Müller N., Keller N, Naguleswaran A., Bohne W., McAllister M., Björkman C., Müller E., Caldelari R. and Hemphill A. 2002 Exogenous nitric oxide triggers Neospora caninum tachyzoite-to-bradyzoite stage conversion in murine epidermal keratinocyte cell cultures. Int. J. Parasitol. 32, 1253-1265.
- Müller N., Vonlaufen N., Gianinazzi G., Leib S. L., and Hemphill A. 2002. Application of real time fluorescent PCR for quantitative assessment of Neospora caninum infections in organotypic slice cultures of rat central nervous tissue. J. Clin. Microbiol 40, 252-255.
- Hemphill A., Vonlaufen N., Naguleswaran A., Keller N., Riesen M., Guetg N, Srinivasan S. and Alaeddine F. 2003. Tissue culture and explant approaches to study and visualize Neospora caninum and its interactions with the host cell. Microscopy and Microanalyses: in press
| [*] | Neospora caninum and neosporosisNeospora caninum (Apicomplexa: Eimeriina: Sarcocystidae) is a Toxoplasma-like parasite that was first identified in 1984 in dogs with encephalomyelitis and myositis. It was later also reported in various species of livestock, especially cattle. In the USA, the EU, and many other countries worldwide, neosporosis is the leading diagnosed cause of abortion in cattle (Ref. 1). One possible route of transmission in cattle (= intermediate host) is through the oral uptake of sporozoite-containing oocysts (see Fig. 1) which are shed in the faeces of infected dogs (= definitive host). Sporozoites enter the intestinal epithelium of cattle and other intermediate hosts, infect macrophages and lymphocytes, and disseminate throughout the body. They transform to the rapidly proliferating and disease-causing tachyzoite stage. Subsequently, the host immune response and possibly other factors trigger stage conversion of the parasite to the slowly proliferating N. caninum bradyzoites, which forms intracellular cysts surrounded by a solid cyst wall. Bradyzoites can survive within a latently infected but immuno-competent animal for many years without causing any clinical symptoms. Reactivation of bradyzoites, which involves stage conversion to tachyzoites, during immuno-suppression (e.g. during pregnancy) can lead to severe disease. N. caninum can only proliferate and survive during most stages of its life cycle as an intracellular parasite, and it is the tachyzoite-to-bradyzoite stage conversion process which enables the parasite to survive within its host for extended periods of time. The corresponding molecular mechanisms governing stage conversion need to be elucidated. |



 Fig. 1: Life cycle of the parasite Neospora caninum.
Fig. 1: Life cycle of the parasite Neospora caninum.

